The Evidence for Effective Inhibition of INa Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid), a Known Blocker of CaV Channels
- PMID: 35409204
- PMCID: PMC8998350
- DOI: 10.3390/ijms23073845
The Evidence for Effective Inhibition of INa Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid), a Known Blocker of CaV Channels
Abstract
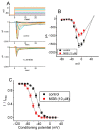
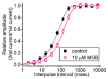
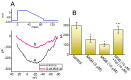
Mirogabalin (MGB, Tarlige®), an inhibitor of the α2δ-1 subunit of voltage-gated Ca2+ (CaV) channels, is used as a way to alleviate peripheral neuropathic pain and diabetic neuropathy. However, to what extent MGB modifies the magnitude, gating, and/or hysteresis of various types of plasmalemmal ionic currents remains largely unexplored. In pituitary tumor (GH3) cells, we found that MGB was effective at suppressing the peak (transient, INa(T)) and sustained (late, INa(L)) components of the voltage-gated Na+ current (INa) in a concentration-dependent manner, with an effective IC50 of 19.5 and 7.3 μM, respectively, while the KD value calculated on the basis of minimum reaction scheme was 8.2 μM. The recovery of INa(T) inactivation slowed in the presence of MGB, although the overall current-voltage relation of INa(T) was unaltered; however, there was a leftward shift in the inactivation curve of the current. The magnitude of the window (INa(W)) or resurgent INa (INa(R)) evoked by the respective ascending or descending ramp pulse (Vramp) was reduced during cell exposure to MGB. MGB-induced attenuation in INa(W) or INa(R) was reversed by the further addition of tefluthrin, a pyrethroid insecticide known to stimulate INa. MGB also effectively lessened the strength of voltage-dependent hysteresis of persistent INa in response to the isosceles triangular Vramp. The cumulative inhibition of INa(T), evoked by pulse train stimulation, was enhanced in its presence. Taken together, in addition to the inhibition of CaV channels, the NaV channel attenuation produced by MGB might have an impact in its analgesic effects occurring in vivo.
Keywords: current kinetics; hysteresis; mirogabalin (Tarlige®, 1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid); persistent Na+ current; pulse train stimulation; resurgent Na+ current; voltage-gated Na+ current; window Na+ current.
Conflict of interest statement
The authors have no conflicts of interest to disclose. The content and writing of this paper are solely the responsibility of the authors.
Figures






Similar articles
-
Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line.Int J Mol Sci. 2022 Jul 17;23(14):7892. doi: 10.3390/ijms23147892. Int J Mol Sci. 2022. PMID: 35887240 Free PMC article.
-
Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents.Int J Mol Sci. 2022 Aug 21;23(16):9453. doi: 10.3390/ijms23169453. Int J Mol Sci. 2022. PMID: 36012718 Free PMC article. Review.
-
Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process.Int J Mol Sci. 2023 Jan 16;24(2):1805. doi: 10.3390/ijms24021805. Int J Mol Sci. 2023. PMID: 36675319 Free PMC article.
-
Concerted suppressive effects of carisbamate, an anti-epileptic alkyl-carbamate drug, on voltage-gated Na+ and hyperpolarization-activated cation currents.Front Cell Neurosci. 2023 May 24;17:1159067. doi: 10.3389/fncel.2023.1159067. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37293624 Free PMC article.
-
Mirogabalin besylate in the treatment of neuropathic pain.Drugs Today (Barc). 2020 Feb;56(2):135-149. doi: 10.1358/dot.2020.56.2.3100504. Drugs Today (Barc). 2020. PMID: 32163529 Review.
Cited by
-
Effective Modulation by Lacosamide on Cumulative Inhibition of INa during High-Frequency Stimulation and Recovery of INa Block during Conditioning Pulse Train.Int J Mol Sci. 2022 Oct 8;23(19):11966. doi: 10.3390/ijms231911966. Int J Mol Sci. 2022. PMID: 36233266 Free PMC article.
-
Safinamide, an inhibitor of monoamine oxidase, modulates the magnitude, gating, and hysteresis of sodium ion current.BMC Pharmacol Toxicol. 2024 Feb 8;25(1):17. doi: 10.1186/s40360-024-00739-5. BMC Pharmacol Toxicol. 2024. PMID: 38331833 Free PMC article.
-
Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line.Int J Mol Sci. 2022 Jul 17;23(14):7892. doi: 10.3390/ijms23147892. Int J Mol Sci. 2022. PMID: 35887240 Free PMC article.
-
Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents.Int J Mol Sci. 2022 Aug 21;23(16):9453. doi: 10.3390/ijms23169453. Int J Mol Sci. 2022. PMID: 36012718 Free PMC article. Review.
-
Characterization in Effective Stimulation on the Magnitude, Gating, Frequency Dependence, and Hysteresis of INa Exerted by Picaridin (or Icaridin), a Known Insect Repellent.Int J Mol Sci. 2022 Aug 26;23(17):9696. doi: 10.3390/ijms23179696. Int J Mol Sci. 2022. PMID: 36077093 Free PMC article.
References
-
- Domon Y., Kitano Y., Makino M. Analgesic effects of the novel α2δ ligand mirogabalin in a rat model of spinal cord injury. Pharmazie. 2018;73:659–661. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

