Metastasis and MAPK Pathways
- PMID: 35409206
- PMCID: PMC8998814
- DOI: 10.3390/ijms23073847
Metastasis and MAPK Pathways
Abstract
Cancer is a leading cause of death worldwide. In many cases, the treatment of the disease is limited due to the metastasis of cells to distant locations of the body through the blood and lymphatic drainage. Most of the anticancer therapeutic options focus mainly on the inhibition of tumor cell growth or the induction of cell death, and do not consider the molecular basis of metastasis. The aim of this work is to provide a comprehensive review focusing on cancer metastasis and the mitogen-activated protein kinase (MAPK) pathway (ERK/JNK/P38 signaling) as a crucial modulator of this process.
Keywords: c-JUN N-terminal kinase (JNK); cancer; extracellular signal-regulated kinase (ERK); metastasis; mitogen-activated kinases (MAPKs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

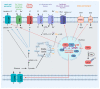


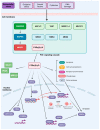
References
-
- Eyles J., Puaux A.-L., Wang X., Toh B., Prakash C., Hong M., Tan T.G., Zheng L., Ong L.C., Jin Y., et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Investig. 2010;120:2030–2039. doi: 10.1172/JCI42002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

