The Interplay between PARP Inhibitors and Immunotherapy in Ovarian Cancer: The Rationale behind a New Combination Therapy
- PMID: 35409229
- PMCID: PMC8998760
- DOI: 10.3390/ijms23073871
The Interplay between PARP Inhibitors and Immunotherapy in Ovarian Cancer: The Rationale behind a New Combination Therapy
Abstract
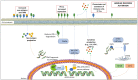
Ovarian cancer (OC) has a high impact on morbidity and mortality in the female population. Survival is modest after platinum progression. Therefore, the search for new therapeutic strategies is of utmost importance. BRCA mutations and HR-deficiency occur in around 50% of OC, leading to increased response and survival after Poly (ADP-ribose) polymerase inhibitors (PARPis) administration. PARPis represent a breakthrough for OC therapy, with three different agents approved. On the contrary, immune checkpoint inhibitors (ICIs), another breakthrough therapy for many solid tumors, led to modest results in OC, without clinical approvals and even withdrawal of clinical trials. Therefore, combinations aiming to overcome resistance mechanisms have become of great interest. Recently, PARPis have been evidenced to modulate tumor microenvironment at the molecular and cellular level, potentially enhancing ICIs responsiveness. This represents the rationale for the combined administration of PARPis and ICIs. Our review ought to summarize the preclinical and translational features that support the contemporary administration of these two drug classes, the clinical trials conducted so far, and future directions with ongoing studies.
Keywords: BRCA; HRD; ICIs; OC; PARP inhibitors; durvalumab; immune checkpoint inhibitors; niraparib; olaparib; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest with regard to this manuscript.
Figures

References
-
- Globocan 2020. Ovary. [(accessed on 15 June 2021)]. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf.
-
- Piccart M.J., Bertelsen K., James K., Cassidy J., Mangioni C., Simonsen E., Stuart G., Kaye S., Vergote I., Blom R., et al. Randomized Intergroup Trial of Cisplatin-Paclitaxel Versus Cisplatin-Cyclophosphamide in Women With Advanced Epithelial Ovarian Cancer: Three-Year Results. J. Nat. Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. - DOI - PubMed
-
- Ozols R.F., Bundy B.N., Greer B.E., Fowler J.M., Clarke-Pearson D., Burger R.A., Mannel R.S., DeGeest K., Hartenbach E.M., Baergen R. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. JCO. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

