Curcuminoids Inhibit Angiogenic Behaviors of Human Umbilical Vein Endothelial Cells via Endoglin/Smad1 Signaling
- PMID: 35409247
- PMCID: PMC8998963
- DOI: 10.3390/ijms23073889
Curcuminoids Inhibit Angiogenic Behaviors of Human Umbilical Vein Endothelial Cells via Endoglin/Smad1 Signaling
Abstract
Background: Angiogenesis is primarily attributed to the excessive proliferation and migration of endothelial cells. Targeting the vascular endothelial growth factor (VEGF) is therefore significant in anti-angiogenic therapy. Although these treatments have not reached clinical expectations, the upregulation of alternative angiogenic pathways (endoglin/Smad1) may play a critical role in drug (VEGF-neutralizing agents) resistance. Enhanced endoglin expression following a VEGF-neutralizing therapy (semaxanib®) was noted in patients. Treatment with an endoglin-targeting antibody augmented VEGF expression in human umbilical vein endothelial cells (HUVECs). Therefore, approaches that inhibit both the androgen and VEGF pathways enhance the HUVECs cytotoxicity and reverse semaxanib resistance. The purpose of this study was to find natural-occurring compounds that inhibited the endoglin-targeting pathway.
Methods: Curcuminoids targeting endoglin were recognized from two thousand compounds in the Traditional Chinese Medicine Database@Taiwan (TCM Database@Taiwan) using Discovery Studio 4.5.
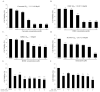
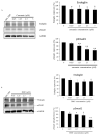
Results: Our results, obtained using cytotoxicity, migration/invasion, and flow cytometry assays, showed that curcumin (Cur) and demethoxycurcumin (DMC) reduced angiogenesis. In addition, Cur and DMC downregulated endoglin/pSmad1 phosphorylation.
Conclusions: The study first showed that Cur and DMC demonstrated antiangiogenic activity via the inhibition of endoglin/Smad1 signaling. Synergistic effects of curcuminoids (i.e., curcumin and DMC) and semaxanib on HUVECs were found. This might be attributed to endoglin/pSmad1 downregulation in HUVECs. Combination treatment with curcuminoids and a semaxanib is therefore expected to reverse semaxanib resistance.
Keywords: Smad1; VEGF; angiogenesis; curcuminoids; endoglin.
Conflict of interest statement
We declare no conflict of interest.
Figures







References
-
- Zangari M., Anaissie E., Stopeck A., Morimoto A., Tan N., Lancet J., Cooper M., Hannah A., Garcia-Manero G., Faderl S., et al. Phase II study of SU5416, a small molecule vascular endothelial growth factor tyrosine kinase receptor inhibitor, in patients with refractory multiple myeloma. Clin. Cancer Res. 2004;10:88–95. doi: 10.1158/1078-0432.CCR-0221-3. - DOI - PubMed
-
- Zhang X., Zhang Y., Jia Y., Qin T., Zhang C., Li Y., Huang C., Liu Z., Wang J., Li K. Bevacizumab promotes active biological behaviors of human umbilical vein endothelial cells by activating TGFbeta1 pathways via off-VEGF signaling. Cancer Biol. Med. 2020;17:418–432. doi: 10.20892/j.issn.2095-3941.2019.0215. - DOI - PMC - PubMed
-
- Fonsatti E., Del Vecchio L., Altomonte M., Sigalotti L., Nicotra M.R., Coral S., Natali P.G., Maio M. Endoglin: An accessory component of the TGF-beta-binding receptor-complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J. Cell. Physiol. 2001;188:1–7. doi: 10.1002/jcp.1095. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

