Exploring the Contribution of Autophagy to the Excess-Sucrose Response in Arabidopsis thaliana
- PMID: 35409249
- PMCID: PMC8999498
- DOI: 10.3390/ijms23073891
Exploring the Contribution of Autophagy to the Excess-Sucrose Response in Arabidopsis thaliana
Abstract
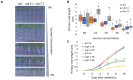
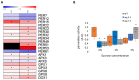
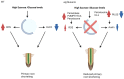
Autophagy is an essential intracellular eukaryotic recycling mechanism, functioning in, among others, carbon starvation. Surprisingly, although autophagy-deficient plants (atg mutants) are hypersensitive to carbon starvation, metabolic analysis revealed that they accumulate sugars under such conditions. In plants, sugars serve as both an energy source and as signaling molecules, affecting many developmental processes, including root and shoot formation. We thus set out to understand the interplay between autophagy and sucrose excess, comparing wild-type and atg mutant seedlings. The presented work showed that autophagy contributes to primary root elongation arrest under conditions of exogenous sucrose and glucose excess but not during fructose or mannitol treatment. Minor or no alterations in starch and primary metabolites were observed between atg mutants and wild-type plants, indicating that the sucrose response relates to its signaling and not its metabolic role. Extensive proteomic analysis of roots performed to further understand the mechanism found an accumulation of proteins essential for ROS reduction and auxin maintenance, which are necessary for root elongation, in atg plants under sucrose excess. The analysis also suggested mitochondrial and peroxisomal involvement in the autophagy-mediated sucrose response. This research increases our knowledge of the complex interplay between autophagy and sugar signaling in plants.
Keywords: Arabidopsis thaliana; autophagy; mitochondria; peroxisome; reactive oxygen species (ROS); sucrose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Avin-Wittenberg T., Baluška F., Bozhkov P.V., Elander P.H., Fernie A.R., Galili G., Hassan A., Hofius D., Isono E., Le Bars R., et al. Autophagy-related approaches for improving nutrient use efficiency and crop yield protection. J. Exp. Bot. 2018;69:1335–1353. doi: 10.1093/jxb/ery069. - DOI - PubMed
-
- Barros J.A.S., Cavalcanti J.H.F., Medeiros D.B., Nunes-Nesi A., Avin-Wittenberg T., Fernie A.R., Araujo W.L. Autophagy deficiency compromises alternative pathways of respiration following energy deprivation in Arabidopsis thaliana. Plant Physiol. 2017;175:62–76. doi: 10.1104/pp.16.01576. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

