Sex Differences in Cardiovascular Diseases: A Matter of Estrogens, Ceramides, and Sphingosine 1-Phosphate
- PMID: 35409368
- PMCID: PMC8999971
- DOI: 10.3390/ijms23074009
Sex Differences in Cardiovascular Diseases: A Matter of Estrogens, Ceramides, and Sphingosine 1-Phosphate
Abstract
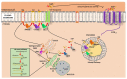
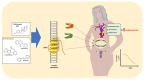
The medical community recognizes sex-related differences in pathophysiology and cardiovascular disease outcomes (CVD), culminating with heart failure. In general, pre-menopausal women tend to have a better prognosis than men. Explaining why this occurs is not a simple matter. For decades, sex hormones like estrogens (Es) have been identified as one of the leading factors driving these sex differences. Indeed, Es seem protective in women as their decline, during and after menopause, coincides with an increased CV risk and HF development. However, clinical trials demonstrated that E replacement in post-menopause women results in adverse cardiac events and increased risk of breast cancer. Thus, a deeper understanding of E-related mechanisms is needed to provide a vital gateway toward better CVD prevention and treatment in women. Of note, sphingolipids (SLs) and their metabolism are strictly related to E activities. Among the SLs, ceramide and sphingosine 1-phosphate play essential roles in mammalian physiology, particularly in the CV system, and appear differently modulated in males and females. In keeping with this view, here we explore the most recent experimental and clinical observations about the role of E and SL metabolism, emphasizing how these factors impact the CV system.
Keywords: cardiovascular; estrogens; sex differences; sphingolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cesaroni G., Mureddu G.F., Agabiti N., Mayer F., Stafoggia M., Forastiere F., Latini R., Masson S., Davoli M., Boccanelli A., et al. Sex differences in factors associated with heart failure and diastolic left ventricular dysfunction: A cross-sectional population-based study. BMC Public Health. 2021;21:415. doi: 10.1186/s12889-021-10442-3. - DOI - PMC - PubMed
-
- DeLeon-Pennell K.Y., Mouton A.J., Ero O.K., Ma Y., Padmanabhan Iyer R., Flynn E.R., Espinoza I., Musani S.K., Vasan R.S., Hall M.E., et al. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res. Cardiol. 2018;113:40. doi: 10.1007/s00395-018-0699-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

