A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury
- PMID: 35409370
- PMCID: PMC9000186
- DOI: 10.3390/ijms23074010
A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury
Abstract
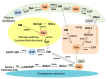
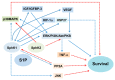
Reactive oxygen species (ROS) modulate sphingolipid metabolism, including enzymes that generate ceramide and sphingosine-1-phosphate (S1P), and a ROS-antioxidant rheostat determines the metabolism of ceramide-S1P. ROS induce ceramide production by activating ceramide-producing enzymes, leading to apoptosis, while they inhibit S1P production, which promotes survival by suppressing sphingosine kinases (SphKs). A ceramide-S1P rheostat regulates ROS-induced mitochondrial dysfunction, apoptotic/anti-apoptotic Bcl-2 family proteins and signaling pathways, leading to apoptosis, survival, cell proliferation, inflammation and fibrosis in the kidney. Ceramide inhibits the mitochondrial respiration chain and induces ceramide channel formation and the closure of voltage-dependent anion channels, leading to mitochondrial dysfunction, altered Bcl-2 family protein expression, ROS generation and disturbed calcium homeostasis. This activates ceramide-induced signaling pathways, leading to apoptosis. These events are mitigated by S1P/S1P receptors (S1PRs) that restore mitochondrial function and activate signaling pathways. SphK1 promotes survival and cell proliferation and inhibits inflammation, while SphK2 has the opposite effect. However, both SphK1 and SphK2 promote fibrosis. Thus, a ceramide-SphKs/S1P rheostat modulates oxidant-induced kidney injury by affecting mitochondrial function, ROS production, Bcl-2 family proteins, calcium homeostasis and their downstream signaling pathways. This review will summarize the current evidence for a role of interaction between ROS-antioxidants and ceramide-SphKs/S1P and of a ceramide-SphKs/S1P rheostat in the regulation of oxidative stress-mediated kidney diseases.
Keywords: Bcl-2 family proteins; apoptosis; ceramide; fibrosis; inflammation; kidney injury; mitochondria; proliferation; reactive oxygen species; sphingosine-1-phosphate.
Conflict of interest statement
The author declares no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

