The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology
- PMID: 35409380
- PMCID: PMC8999875
- DOI: 10.3390/ijms23074022
The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology
Abstract
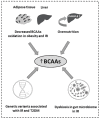
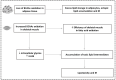
Branched chain amino acids (BCAAs), leucine, isoleucine and valine, are essential amino acids widely studied for their crucial role in the regulation of protein synthesis mainly through the activation of the mTOR signaling pathway and their emerging recognition as players in the regulation of various physiological and metabolic processes, such as glucose homeostasis. BCAA supplementation is primarily used as a beneficial nutritional intervention in chronic liver and kidney disease as well as in muscle wasting disorders. However, downregulated/upregulated plasma BCAAs and their defective catabolism in various tissues, mainly due to altered enzymatic activity of the first two enzymes in their catabolic pathway, BCAA aminotransferase (BCAT) and branched-chain α-keto acid dehydrogenase (BCKD), have been investigated in many nutritional and disease states. The current review focused on the underlying mechanisms of altered BCAA catabolism and its contribution to the pathogenesis of a numerous pathological conditions such as diabetes, heart failure and cancer. In addition, we summarize findings that indicate that the recovery of the dysregulated BCAA catabolism may be associated with an improved outcome and the prevention of serious disease complications.
Keywords: BCKAs; T2DM; cancer; catabolic enzymes; catabolism; heart failure; isoleucine; leucine; valine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

