The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment
- PMID: 35409403
- PMCID: PMC8999506
- DOI: 10.3390/ijms23074039
The Porcine Odorant-Binding Protein as a Probe for an Impedenziometric-Based Detection of Benzene in the Environment
Abstract
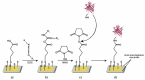
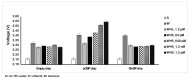
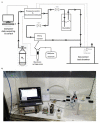
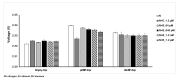
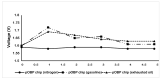
Odorant-binding proteins (OBPs) are a group of small and soluble proteins present in both vertebrates and insects. They have a high level of structural stability and bind to a large spectrum of odorant molecules. In the environmental field, benzene is the most dangerous compound among the class of pollutants named BTEX (benzene, toluene, ethylbenzene, and xylene). It has several effects on human health and, consequently, it appears to be important to monitor its presence in the environment. Commonly, its detection requires the use of very sophisticated and time-consuming analytical techniques (GC-MS, etc.) as well as the presence of specialized personnel. Here, we present the application of an odorant-binding protein (pOBP) isolated from pigs as a molecular recognition element (MRE) for a low-energy impedenziometric biosensor for outdoor and real-time benzene detection. The obtained results show that the biosensor can detect the presence of 64 pM (5 µg/m3) benzene, the limit value of exposure for human health set by the European Directive 2008/50/EC.
Keywords: VOCs; benzene; biosensors; odorant-binding protein (OBP).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

