Sclerocarya birrea (Marula) Extract Inhibits Hepatic Steatosis in db/db Mice
- PMID: 35409465
- PMCID: PMC8998011
- DOI: 10.3390/ijerph19073782
Sclerocarya birrea (Marula) Extract Inhibits Hepatic Steatosis in db/db Mice
Abstract
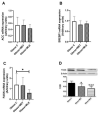
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of hepatic metabolic perturbations ranging from simple steatosis to steatohepatitis, cirrhosis and hepatocellular carcinoma. Currently, lifestyle modifications to reduce weight gain are considered the most effective means of preventing and treating the disease. The aim of the present study was to determine the therapeutic benefit of Sclerocarya birrea (Marula leaf extract, MLE) on hepatic steatosis. Obese db/db mice were randomly stratified into the obese control, metformin (MET) or MLE-treated groups. Mice were treated daily for 29 days, at which point all mice were euthanized and liver samples were collected. Hematoxylin and eosin staining was used for histological assessment of the liver sections, while qRT-PCR and Western blot were used to determine hepatic mRNA and protein expression, respectively. Thereafter, the association between methylenetetrahydrofolate reductase (Mthfr a key enzyme in one-carbon metabolism and DNA-methylation-induced regulation of gene transcription) and lipogenic genes was evaluated using Pearson's correlation coefficient. Mice treated with MLE presented with significantly lower body and liver weights as compared with the obese control and MET-treated mice (p ≤ 0.05). Further, MLE treatment significantly inhibited hepatic steatosis as compared with the obese control and MET-treated mice (p ≤ 0.05). The reduced lipid accumulation was associated with low expression of fatty acid synthase (Cpt1; p ≤ 0.05) and an upregulation of the fatty acid oxidation gene, carnitine palmitoyltransferase (Cpt1; p ≤ 0.01), as compared with the obese control mice. Interestingly, MLE treatment improved the correlation between Mthfr and Cpt1 mRNA expression (r = 0.72, p ≤ 0.01). Taken together, the results suggest that Marula leaf extracts may inhibit hepatic steatosis by influencing the association between Mthfr and genes involved in hepatic lipid metabolism. Further studies are warranted to assess DNA methylation changes in lipid metabolism genes.
Keywords: DNA methylation; Marula leaf extract; methylenetetrahydrofolate reductase; non-alcoholic fatty liver disease; β-oxidation.
Conflict of interest statement
There is no conflict of interest reported by the authors. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures






References
-
- Jarvis H., Craig D., Barker R., Spiers G., Stow D., Anstee Q.M., Hanratty B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17:e1003100. doi: 10.1371/journal.pmed.1003100. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

