Evaluation of BNT162b2 Vaccine Effectiveness in Galicia, Northwest Spain
- PMID: 35409724
- PMCID: PMC8998680
- DOI: 10.3390/ijerph19074039
Evaluation of BNT162b2 Vaccine Effectiveness in Galicia, Northwest Spain
Abstract
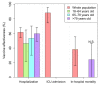
Investigating vaccine effectiveness (VE) in real-world conditions is crucial, especially its variation across different settings and populations. We undertook a test-negative control study in Galicia (Northwest Spain) to assess BNT162b2 effectiveness against acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as well as COVID-19 associated hospitalization, intensive care unit (ICU) admission and mortality. A total of 44,401 positive and 817,025 negative SARS-CoV-2 test results belonging to adults were included. Adjusted odds ratios of vaccination and their 95% confidence interval (CI) were estimated using multivariate logistic-regression models. BNT162b2 showed high effectiveness in reducing SARS-CoV-2 infections in all age categories, reaching maximum VE ≥ 14 days after administering the second dose [18-64 years: VE = 92.9% (95%CI: 90.2-95.1); 65-79 years: VE = 85.8% (95%CI: 77.3-91.9), and ≥80 years: VE = 91.4% (95%CI: 87.9-94.1)]. BNT162b2 also demonstrated effectiveness in preventing COVID-19 hospitalization for all age categories, with VE more pronounced for those aged ≥80 years [VE = 60.0% (95%CI: 49.4-68.3)]. Moreover, there was a considerable reduction in ICU admission [VE = 88.0% (95%CI: 74.6-95.8)] and mortality [VE = 38.0% (95%CI: 15.9-55.4)] in the overall population. BNT162b2 showed substantial protection against SARS-CoV-2 infections and COVID-19 severity. Our findings would prove useful for systematic reviews and meta-analysis on COVID-19 VE.
Keywords: COVID-19; SARS-CoV-2; population study; vaccine effectiveness.
Conflict of interest statement
F.M.-T. received honoraria from GSK group of companies, Pfizer Inc., Sanofi Pasteur, MSD, Seqirus, and Janssen for taking part in advisory boards and expert meetings and for acting as a speaker in congresses outside the scope of the submitted work. F.M.-T. has also acted as principal investigator in randomized controlled trials of the above-mentioned companies as well as Ablynx, Gilead, Regeneron, Roche, Abbott, Novavax, and MedImmune, with honoraria paid to his institution. The authors declare no conflict of interest.
Figures

References
-
- European Medicines Agency . COVID-19 Vaccines: Authorised. European Medicines Agency; Amsterdam, The Netherlands: 2020.
-
- Ministerio de Sanidad . Estrategia de Vacunación Frente al COVD-19 en España. Ministerio de Sanidad; Madrid, Spain: 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

