Proposal for Handling of Medicine Shortages Based on a Comparison of Retrospective Risk Analysis
- PMID: 35409787
- PMCID: PMC8998354
- DOI: 10.3390/ijerph19074102
Proposal for Handling of Medicine Shortages Based on a Comparison of Retrospective Risk Analysis
Abstract
Introduction: We reviewed and compared current drug shortages and shortage management practices in six selected countries (Hungary, Belgium, Spain, Switzerland, Australia, United States) based on the most comprehensive national shortage databases for each country, for four Anatomical Therapeutic Chemical (ATC) groups, to analyze the criticality of drug shortages across countries and identify best practices in shortage management strategies.
Materials and methods: Countries were selected to cover a wide geographical range of high-income nations where a lack of economic power as a potential source of drug shortages is not observable. ATC groups were selected based on a pre-examination of the databases to analyze groups most often in shortage, and groups where the absence of which could have a severe negative impact on treatment outcomes. The bias originating from the different reporting systems had to be reduced to gain comprehensive and comparable information. The first bias-reducing mechanism was transforming the raw number of shortages into proportion per million people. Secondly, critical cases were classified, and thirdly, critical cases were compared with the Word Health Organization (WHO) Essential Medicine Lists.
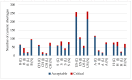
Results: The results indicate that every European country studied reports significantly higher total and critical shortages per population compared to the US and Australia. Within Europe, Hungary reports the highest number of cases both for total and critical shortages, while Spain has the lowest results in both aspects. While in the US and Australia critical shortages were observable in similar proportions across all ATC groups, in European countries ATC groups of anti-infectives for systemic use (J) and the nervous system (N) were found to account for a notably higher proportion of critical shortages. Current shortage management practices were examined in each country and classified into five groups to identify common best practices.
Conclusions: Due to the different characterization of reporting systems, several bias-reducing mechanisms should be applied to compare and evaluate shortages. In addition, European harmonization should be initiated to create mutually acknowledged definitions and reporting systems, which could be the basis of good drug shortage handling practices in Europe.
Keywords: comparison; countermeasures; critical medicines; drug shortage; risk assessment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
National and transnational drug shortages: a quantitative descriptive study of public registers in Europe and the USA.BMC Health Serv Res. 2022 Jul 22;22(1):940. doi: 10.1186/s12913-022-08309-3. BMC Health Serv Res. 2022. PMID: 35869486 Free PMC article.
-
Drug shortages in China: a cross-sectional study.BMC Health Serv Res. 2023 May 4;23(1):438. doi: 10.1186/s12913-023-09295-w. BMC Health Serv Res. 2023. PMID: 37143100 Free PMC article.
-
Drug shortages in European countries: a trade-off between market attractiveness and cost containment?BMC Health Serv Res. 2014 Sep 26;14:438. doi: 10.1186/1472-6963-14-438. BMC Health Serv Res. 2014. PMID: 25257912 Free PMC article.
-
Managing pharmaceutical shortages during the COVID pandemic: An exploratory analysis of European collective and national government responses.J Med Access. 2022 Sep 17;6:27550834221123425. doi: 10.1177/27550834221123425. eCollection 2022 Jan-Dec. J Med Access. 2022. PMID: 36204525 Free PMC article. Review.
-
Medicines Shortages Reporting Systems (MSRS): An exploratory review of access and sustainability.Res Social Adm Pharm. 2024 Jun;20(6):72-83. doi: 10.1016/j.sapharm.2024.02.010. Epub 2024 Feb 20. Res Social Adm Pharm. 2024. PMID: 38458895 Review.
Cited by
-
Price increase negotiations to address drug shortages in South Korea's national health insurance.Front Pharmacol. 2023 Dec 4;14:1307462. doi: 10.3389/fphar.2023.1307462. eCollection 2023. Front Pharmacol. 2023. PMID: 38111380 Free PMC article.
-
Impact of medicine shortages on hospital practice: role of a multidisciplinary medicine shortages team.Int J Clin Pharm. 2024 Dec;46(6):1563-1569. doi: 10.1007/s11096-024-01772-4. Epub 2024 Jul 15. Int J Clin Pharm. 2024. PMID: 39007990
-
Managing Antibiotic Shortages in Inpatient Care-A Review of Recent Years in Comparison with the Hungarian Status.Antibiotics (Basel). 2023 Dec 7;12(12):1704. doi: 10.3390/antibiotics12121704. Antibiotics (Basel). 2023. PMID: 38136738 Free PMC article.
-
Shortages of Medicines to Treat COVID-19 Symptoms during the First Wave and Fourth Wave: Analysis of Notifications Reported to Registers in Austria, Italy, and Spain.Pharmacy (Basel). 2023 Jul 21;11(4):120. doi: 10.3390/pharmacy11040120. Pharmacy (Basel). 2023. PMID: 37489351 Free PMC article.
-
Prevention and management of health products shortages by the French national agency (ANSM), 10 years of experience.Front Public Health. 2023 Nov 17;11:1293110. doi: 10.3389/fpubh.2023.1293110. eCollection 2023. Front Public Health. 2023. PMID: 38045969 Free PMC article.
References
-
- Turbucz B., Hankó B. Overview of the causes and management of drug shortages in the United States and in Hungary. Acta Pharm. Hung. 2020;90:170–184. doi: 10.33892/aph.2020.90.170-184. - DOI
-
- Videau M., Chemali L., Stucki C., Saavedra-Mitjans M., Largana S., Guerin A., Bonnabry P., Delhauteur B., VAN Hees T., Lebel D., et al. Drug Shortages in Canada and Selected European Countries: A Cross-Sectional, Institution-Level Comparison. Can. J. Hosp. Pharm. 2019;72:7–15. doi: 10.4212/cjhp.v72i1.2863. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

