Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment
- PMID: 35409817
- PMCID: PMC8998984
- DOI: 10.3390/ijerph19074134
Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment
Abstract
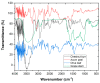
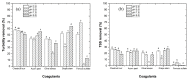
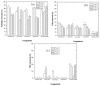
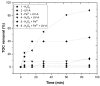
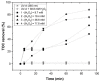
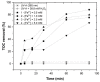
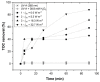
Re-using and adding value to by-products is one of the current focuses of the agri-food industry, following the Sustainable Development Goals of United Nations. In this work, the by-products of four plants, namely chestnut burr, acorn peel, olive leaf, and grape stem were used as coagulants to treat elderberry wastewater (EW), a problematic liquid effluent. EW pre-treatment using these natural coagulants showed promising results after pH and coagulant dosage optimization. However, the decrease in total organic carbon (TOC) was not significant, due to the addition of the plant-based natural coagulants which contain carbon content. After this pre-treatment, the photo-Fenton advanced oxidation process was selected, after preliminary assays, to improve the global performance of the EW treatment. Photo-Fenton was also optimized for the parameters of pH, H2O2, Fe2+, and irradiance power, and the best conditions were applied to the EW treatment. Under the best operational conditions defined in the parametric study, the combined results of coagulation-flocculation-decantation (CFD) and photo-Fenton for chestnut burr, acorn peel, olive leaf, and grape stem were, respectively, 90.2, 89.5, 91.5, and 88.7% for TOC removal; 88.7, 82.0, 90.2 and 93.1%, respectively, for turbidity removal; and finally, 40.6, 42.2, 45.3, and 39.1%, respectively, for TSS removal. As a final remark, it is possible to suggest that plant-based coagulants, combined with photo-Fenton, can be a promising strategy for EW treatment that simultaneously enables valorization by adding value back to food by-products.
Keywords: by-products reuse; circular economy; coagulation–flocculation–decantation; photo-Fenton; plant-based coagulants; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Esposito B., Sessa M.R., Sica D., Malandrino O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability. 2020;12:7401. doi: 10.3390/su12187401. - DOI
-
- United Nations Sustainable Development Goals. [(accessed on 6 December 2021)]. Available online: https://www.un.org/sustainabledevelopment/
-
- Ang W.L., Mohammad A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020;262:121267. doi: 10.1016/j.jclepro.2020.121267. - DOI
-
- Tsani S., Koundouri P., Akinsete E. Resource management and sustainable development: A review of the European water policies in accordance with the United Nations’ Sustainable Development Goals. Environ. Sci. Policy. 2020;114:570–579. doi: 10.1016/j.envsci.2020.09.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

