Efficacy and Sustainability of Diabetes-Specific Meal Replacement on Obese and Overweight Type-2 Diabetes Mellitus Patients: Study Approaches for a Randomised Controlled Trial and Impact of COVID-19 on Trial Progress
- PMID: 35409872
- PMCID: PMC8998339
- DOI: 10.3390/ijerph19074188
Efficacy and Sustainability of Diabetes-Specific Meal Replacement on Obese and Overweight Type-2 Diabetes Mellitus Patients: Study Approaches for a Randomised Controlled Trial and Impact of COVID-19 on Trial Progress
Abstract
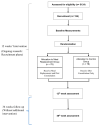
Meal replacement (MR) is widely used in weight and diabetes management programs due to its ease of compliance and handling. However, little is known about its impact on outcomes other than glycaemic control and weight loss. Furthermore, not many studies evaluate its cost-effectiveness and sustainability. This study aimed to evaluate the efficacy of a diabetes-specific MR for the weight reduction and glycaemic controls of overweight and obese T2DM patients, as compared to routine dietary consultation. Other health outcomes, the cost effectiveness, and the sustainability of the MR will also be evaluated. Materials and Methods: This randomised controlled clinical trial will involve 156 participants who have been randomised equally into the intervention and control groups. As a baseline, both groups will receive diet consultation. Additionally, the intervention group will receive an MR to replace one meal for 5 days a week. The duration of intervention will be 12 weeks, with 36 weeks of follow-up to monitor the sustainability of the MR. The primary endpoints are weight and Hemoglobin A1c (HbA1c) reduction, while the secondary endpoints are anthropometry, biochemical measurements, satiety, hormone changes, quality of life, and cost-effectiveness. The impact of the COVID-19 pandemic on study design is also discussed in this paper. This study has obtained human ethics approval from RECUKM (JEP-2019-566) and is registered at the Thai Clinical Trials Registry (TCTR ID: TCTR20210921004).
Keywords: HbA1c; diabetes; diet modification; glycemic control; meal replacement; obesity.
Conflict of interest statement
The authors reported no conflict of interest.
References
-
- World Health Organization . Diabetes. WHO; Geneva, Switzerland: 2020. [(accessed on 3 January 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes.
-
- United Nations World Population Review. 2020. [(accessed on 18 November 2020)]. Available online: https://worldpopulationreview.com/country-rankings/obesity-rates-by-country.
-
- Institute of Public Health Malaysia . National Health and Morbidity Survey 2019. Ministry of Health Malaysia; Kuala Lumpur, Malaysia: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials