Culture Isolate of Rickettsia felis from a Tick
- PMID: 35410003
- PMCID: PMC8998211
- DOI: 10.3390/ijerph19074321
Culture Isolate of Rickettsia felis from a Tick
Abstract
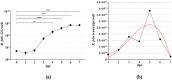
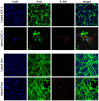
Although the cat flea, Ctenocephalides felis, has been identified as the primary vector of Rickettsia felis, additional flea, tick, mite, and louse species have also been associated with this bacterium by molecular means; however, the role of these arthropods in the transmission of R. felis has not been clarified. Here, we succeeded in culture isolation of R. felis from a host-seeking castor bean tick, Ixodes ricinus, the most common tick in Slovakia. The bacterial isolation was performed on XTC-2 cells at 28 °C using the shell-vial technique. An evaluation of the growth properties was performed for both the XTC-2 and Vero cell lines. We observed R. felis in the infected host cells microscopically by Gimenez staining and immunofluorescence assay. The R. felis isolate was purified by gradient ultracentrifugation and visualized by electron microscopy. Fragments of the genes gltA, ompA, ompB, htrA, rpoB, sca4, rffE, and rrs were amplified and compared with the corresponding sequences of the type strain URRWXCal2 and other R. felis culture -isolated strains. We did not detect any nucleotide polymorphisms; however, plasmid pRFδ, characteristic of the standard strain, was absent in our isolate. Herein, we describe the first successful isolation and characterization of a tick-derived R. felis strain "Danube", obtained from an I. ricinus nymph.
Keywords: Ixodes ricinus; Rickettsia felis; cell culture; shell-vial technique; vector-borne bacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Current Data on Rickettsia felis Occurrence in Vectors, Human and Animal Hosts in Europe: A Scoping Review.Microorganisms. 2022 Dec 16;10(12):2491. doi: 10.3390/microorganisms10122491. Microorganisms. 2022. PMID: 36557744 Free PMC article.
-
Rickettsia vini n. sp. (Rickettsiaceae) infecting the tick Ixodes arboricola (Acari: Ixodidae).Parasit Vectors. 2016 Aug 26;9(1):469. doi: 10.1186/s13071-016-1742-8. Parasit Vectors. 2016. PMID: 27565956 Free PMC article.
-
Isolation of Candidatus Rickettsia vini from Belgian Ixodes arboricola ticks and propagation in tick cell lines.Ticks Tick Borne Dis. 2020 Nov;11(6):101511. doi: 10.1016/j.ttbdis.2020.101511. Epub 2020 Jul 10. Ticks Tick Borne Dis. 2020. PMID: 32993931 Free PMC article.
-
Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line.Appl Environ Microbiol. 2006 Aug;72(8):5589-95. doi: 10.1128/AEM.00532-06. Appl Environ Microbiol. 2006. PMID: 16885313 Free PMC article.
-
Ecology of Rickettsia felis: a review.J Med Entomol. 2009 Jul;46(4):723-36. doi: 10.1603/033.046.0402. J Med Entomol. 2009. PMID: 19645274 Review.
Cited by
-
Rickettsia Deregulates Genes Coding for the Neurotoxic Cell Response Pathways in Cerebrocortical Neurons In Vitro.Cells. 2023 Apr 25;12(9):1235. doi: 10.3390/cells12091235. Cells. 2023. PMID: 37174635 Free PMC article.
-
Current Data on Rickettsia felis Occurrence in Vectors, Human and Animal Hosts in Europe: A Scoping Review.Microorganisms. 2022 Dec 16;10(12):2491. doi: 10.3390/microorganisms10122491. Microorganisms. 2022. PMID: 36557744 Free PMC article.
-
Ticks, Fleas, and Harboured Pathogens from Dogs and Cats in Cyprus.Pathogens. 2022 Nov 23;11(12):1403. doi: 10.3390/pathogens11121403. Pathogens. 2022. PMID: 36558737 Free PMC article.
-
What do we know about the microbiome of I. ricinus?Front Cell Infect Microbiol. 2022 Nov 16;12:990889. doi: 10.3389/fcimb.2022.990889. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36467722 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

