Efficiently generate functional hepatic cells from human pluripotent stem cells by complete small-molecule strategy
- PMID: 35410439
- PMCID: PMC8996222
- DOI: 10.1186/s13287-022-02831-1
Efficiently generate functional hepatic cells from human pluripotent stem cells by complete small-molecule strategy
Abstract
Background: Various methods have been developed to generate hepatic cells from human pluripotent stem cells (hPSCs) that rely on the combined use of multiple expensive growth factors, limiting industrial-scale production and widespread applications. Small molecules offer an attractive alternative to growth factors for producing hepatic cells since they are more economical and relatively stable.
Methods: We dissect small-molecule combinations and identify the ideal cocktails to achieve an optimally efficient and cost-effective strategy for hepatic cells differentiation, expansion, and maturation.
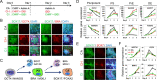
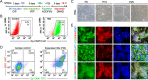
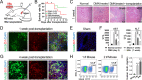
Results: We demonstrated that small-molecule cocktail CIP (including CHIR99021, IDE1, and PD0332991) efficiently induced definitive endoderm (DE) formation via increased endogenous TGF-β/Nodal signaling. Furthermore, we identified that combining Vitamin C, Dihexa, and Forskolin (VDF) could substitute growth factors to induce hepatic specification. The obtained hepatoblasts (HBs) could subsequently expand and mature into functional hepatocyte-like cells (HLCs) by the established chemical formulas. Thus, we established a stepwise strategy with complete small molecules for efficiently producing scalable HBs and functionally matured HLCs. The small-molecule-derived HLCs displayed typical functional characteristics as mature hepatocytes in vitro and repopulating injured liver in vivo.
Conclusion: Our current small-molecule-based hepatic generation protocol presents an efficient and cost-effective platform for the large-scale production of functional human hepatic cells for cell-based therapy and drug discovery using.
Keywords: Hepatic differentiation; Hepatoblasts; Hepatocyte-like cells; Human pluripotent stem cells; Small molecule.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Robust expansion and functional maturation of human hepatoblasts by chemical strategy.Stem Cell Res Ther. 2021 Feb 25;12(1):151. doi: 10.1186/s13287-021-02233-9. Stem Cell Res Ther. 2021. PMID: 33632328 Free PMC article.
-
Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails.Stem Cell Res Ther. 2018 Mar 9;9(1):58. doi: 10.1186/s13287-018-0794-4. Stem Cell Res Ther. 2018. PMID: 29523187 Free PMC article.
-
Small-Molecule-Directed Hepatocyte-Like Cell Differentiation of Human Pluripotent Stem Cells.Curr Protoc Stem Cell Biol. 2016 Aug 17;38:1G.6.1-1G.6.18. doi: 10.1002/cpsc.13. Curr Protoc Stem Cell Biol. 2016. PMID: 27532814
-
An Extracellular Matrix-based Culture System for Generation of Human Pluripotent Stem Cell Derived-hepatocytes.Curr Stem Cell Res Ther. 2021;16(7):888-896. doi: 10.2174/1574888X16666201228144834. Curr Stem Cell Res Ther. 2021. PMID: 33371861 Review.
-
[Generation of Zone-specific Hepatocyte-like Cells from Human Induced Pluripotent Stem Cells for Accurate Prediction of Drug-induced Hepatotoxicity].Yakugaku Zasshi. 2019;139(12):1509-1512. doi: 10.1248/yakushi.19-00170. Yakugaku Zasshi. 2019. PMID: 31787637 Review. Japanese.
Cited by
-
Three-dimensional human bile duct formation from chemically induced human liver progenitor cells.Front Bioeng Biotechnol. 2023 Aug 21;11:1249769. doi: 10.3389/fbioe.2023.1249769. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37671190 Free PMC article.
-
Comparative analysis of small molecule and growth factor-derived human induced pluripotent stem cell-derived hepatocyte-like cells.Front Cell Dev Biol. 2025 Jun 26;13:1594340. doi: 10.3389/fcell.2025.1594340. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40641603 Free PMC article.
-
Hepatic Lipoprotein Metabolism: Current and Future In Vitro Cell-Based Systems.Biomolecules. 2025 Jul 2;15(7):956. doi: 10.3390/biom15070956. Biomolecules. 2025. PMID: 40723828 Free PMC article. Review.
-
A live-cell image-based machine learning strategy for reducing variability in PSC differentiation systems.Cell Discov. 2023 Jun 6;9(1):53. doi: 10.1038/s41421-023-00543-1. Cell Discov. 2023. PMID: 37280224 Free PMC article.
-
Chemical approaches targeting the hurdles of hepatocyte transplantation: mechanisms, applications, and advances.Front Cell Dev Biol. 2024 Oct 31;12:1480226. doi: 10.3389/fcell.2024.1480226. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39544361 Free PMC article. Review.
References
-
- Ang LT, Tan AKY, Autio MI, Goh SH, Choo SH, Lee KL, Tan J, Pan B, Lee JJH, Lum JJ, Lim CYY, Yeo IKX, Wong CJY, Liu M, Oh JLL, Chia CPL, Loh CH, Chen A, Chen Q, Weissman IL, Loh KM, Lim B. A roadmap for human liver differentiation from pluripotent stem cells. Cell Rep. 2018;22(8):2190–2205. - PMC - PubMed
-
- Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, Lee HJ, Kim J, Jung CR, Chung KS, Son MJ. Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 2019;71(5):970–985. - PubMed
-
- Feng S, Wu J, Qiu WL, Yang L, Deng X, Zhou Y, Chen Y, Li X, Yu L, Li H, Xu ZR, Xiao Y, Ren X, Zhang L, Wang C, Sun Z, Wang J, Ding X, Chen Y, Gadue P, Pan G, Ogawa M, Ogawa S, Na J, Zhang P, Hui L, Yin H, Chen L, Xu CR, Cheng X. Large-scale generation of functional and transplantable hepatocytes and cholangiocytes from human endoderm stem cells. Cell Rep. 2020;33(10):108455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

