Schizophrenia Imaging Signatures and Their Associations With Cognition, Psychopathology, and Genetics in the General Population
- PMID: 35410495
- PMCID: PMC9444886
- DOI: 10.1176/appi.ajp.21070686
Schizophrenia Imaging Signatures and Their Associations With Cognition, Psychopathology, and Genetics in the General Population
Abstract
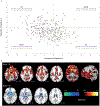
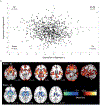
Objective: The prevalence and significance of schizophrenia-related phenotypes at the population level is debated in the literature. Here, the authors assessed whether two recently reported neuroanatomical signatures of schizophrenia-signature 1, with widespread reduction of gray matter volume, and signature 2, with increased striatal volume-could be replicated in an independent schizophrenia sample, and investigated whether expression of these signatures can be detected at the population level and how they relate to cognition, psychosis spectrum symptoms, and schizophrenia genetic risk.
Methods: This cross-sectional study used an independent schizophrenia-control sample (N=347; ages 16-57 years) for replication of imaging signatures, and then examined two independent population-level data sets: typically developing youths and youths with psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort (N=359; ages 16-23 years) and adults in the UK Biobank study (N=836; ages 44-50 years). The authors quantified signature expression using support-vector machine learning and compared cognition, psychopathology, and polygenic risk between signatures.
Results: Two neuroanatomical signatures of schizophrenia were replicated. Signature 1 but not signature 2 was significantly more common in youths with psychosis spectrum symptoms than in typically developing youths, whereas signature 2 frequency was similar in the two groups. In both youths and adults, signature 1 was associated with worse cognitive performance than signature 2. Compared with adults with neither signature, adults expressing signature 1 had elevated schizophrenia polygenic risk scores, but this was not seen for signature 2.
Conclusions: The authors successfully replicated two neuroanatomical signatures of schizophrenia and describe their prevalence in population-based samples of youths and adults. They further demonstrated distinct relationships of these signatures with psychosis symptoms, cognition, and genetic risk, potentially reflecting underlying neurobiological vulnerability.
Keywords: Genetics/Genomics; Machine Learning; Neuroanatomy; Neuroimaging; Polygenic Risk Scores; Schizophrenia Spectrum and Other Psychotic Disorders.
Figures



Comment in
-
Broadening the Parameters of Clinical High Risk for Psychosis.Am J Psychiatry. 2022 Sep;179(9):593-595. doi: 10.1176/appi.ajp.20220612. Am J Psychiatry. 2022. PMID: 36048484 No abstract available.
References
-
- Crespo-Facorro B, Such P, Nylander A-G, et al.: The burden of disease in early schizophrenia: a systematic literature review. Curr Med Res Opin 2020; 37:109–121 - PubMed
-
- Kapur S, Phillips AG, Insel TR: Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry 2012; 17:1174–1179 - PubMed

