Wharton's Jelly-Mesenchymal Stem Cell-Engineered Conduit for Pulmonary Artery Reconstruction in Growing Piglets
- PMID: 35411313
- PMCID: PMC8993765
- DOI: 10.1016/j.jacbts.2021.11.013
Wharton's Jelly-Mesenchymal Stem Cell-Engineered Conduit for Pulmonary Artery Reconstruction in Growing Piglets
Abstract
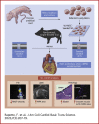
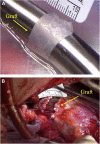
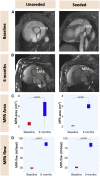
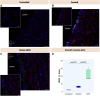
Surgical treatment of congenital heart defects affecting the right ventricular outflow tract often requires complex reconstruction and multiple reoperations. With a randomized controlled trial, we compared a novel tissue-engineered small intestine submucosa-based graft for pulmonary artery reconstruction (seeded with mesenchymal stem cells derived from Wharton's Jelly) with conventional small intestine submucosa in growing piglets. Six months after implantation, seeded grafts showed integration with host tissues at cellular level and exhibited growth potential on transthoracic echocardiography and cardiovascular magnetic resonance. Our seeded graft is a promising biomaterial for pulmonary artery reconstruction in pediatric patients with right ventricular outflow tract abnormalities.
Keywords: CMR, cardiovascular magnetic resonance; FISH, fluorescent in situ hybridization; MPA, main pulmonary artery; RVOT, right ventricular outflow tract; SIS, small intestinal submucosa; SMA, smooth muscle actin; TTE, transthoracic echocardiography; WJ-MSC, Wharton’s Jelly–mesenchymal stem cells; growing swine model; right ventricular outflow tract reconstruction; small intestinal submucosa; tissue engineering.
© 2022 The Authors.
Conflict of interest statement
This study was supported by grants from the Sir Jules Thorn Charitable Trust, the Enid Linder Foundation, and the British Heart Foundation. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures


References
-
- Erikssen G., Liestøl K., Seem E., et al. Achievements in congenital heart defect surgery. Circulation. 2015;131:337–346. - PubMed
-
- Raissadati A., Nieminen H., Haukka J., Sairanen H., Jokinen E. Late causes of death after pediatric cardiac surgery. J Am Coll Cardiol. 2016;68:487–498. - PubMed
-
- Selamet Tierney E.S., Gersony W.M., Altmann K., et al. Pulmonary position cryopreserved homografts: durability in pediatric Ross and non-Ross patients. J Thorac Cardiovasc Surg. 2005;130:282–286. - PubMed
-
- Baskett R.J., Nanton M.A., Warren A.E., Ross D.B. Human leukocyte antigen-DR and ABO mismatch are associated with accelerated homograft valve failure in children: implications for therapeutic interventions. J Thorac Cardiovasc Surg. 2003;126:232–238. - PubMed
-
- Mercer C.W., West S.C., Sharma M.S., Yoshida M., Morell V.O. Polytetrafluoroethylene conduits versus homografts for right ventricular outflow tract reconstruction in infants and young children: an institutional experience. J Thorac Cardiovasc Surg. 2018;155:2082–2091. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

