Measurement of erythrocyte membrane mannoses to assess splenic function
- PMID: 35411940
- PMCID: PMC9321840
- DOI: 10.1111/bjh.18164
Measurement of erythrocyte membrane mannoses to assess splenic function
Abstract
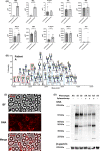
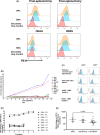
Red blood cells (RBCs) lose plasma membrane in the spleen as they age, but the cells and molecules involved are yet to be identified. Sickle cell disease and infection by Plasmodium falciparum cause oxidative stress that induces aggregates of cross-linked proteins with N-linked high-mannose glycans (HMGs). These glycans can be recognised by mannose-binding lectins, including the mannose receptor (CD206), expressed on macrophages and specialised phagocytic endothelial cells in the spleen to mediate the extravascular haemolysis characteristic of these diseases. We postulated this system might also mediate removal of molecules and membrane in healthy individuals. Surface expression of HMGs on RBCs from patients who had previously undergone splenectomy was therefore assessed: high levels were indeed observable as large membrane aggregates. Glycomic analysis by mass spectrometry identified a mixture of Man5-9 GlcNAc2 structures. HMG levels correlated well with manual pit counts (r = 0.75-0.85). To assess further whether HMGs might act as a splenic reticuloendothelial function test, we measured levels on RBCs from patients with potential functional hyposplenism, some of whom exhibited high levels that may indicate risk of complications.
Keywords: glycosylation; membrane editing; oxidative damage; red blood cells; splenectomy.
© 2022 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Figures

References
-
- Crosby WH. Normal functions of the spleen relative to red blood cells: a review. Blood. 1959. April 01;14(4):399–408. - PubMed
-
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005. August 01;5(8):606–16. - PubMed
-
- Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998. February 01;80(2):173–95. - PubMed
-
- Lutz HU. Innate immune and non‐immune mediators of erythrocyte clearance. Cell Mol Biol (Noisy‐le‐Grand). 2004. March 01;50(2):107–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

