Age-adjusted mortality and predictive value of liver chemistries in a Viennese cohort of COVID-19 patients
- PMID: 35412018
- PMCID: PMC9115240
- DOI: 10.1111/liv.15274
Age-adjusted mortality and predictive value of liver chemistries in a Viennese cohort of COVID-19 patients
Abstract
Background and aims: The coronavirus disease of 2019 (COVID-19) causes considerable mortality worldwide. We aimed to investigate the frequency and predictive role of abnormal liver chemistries in different age groups.
Methods: Patients with positive severe acute respiratory distress syndrome-coronavirus-2 (SARS-CoV-2) polymerase chain reaction (PCR) test between 03/2020-07/2021 at the Vienna General Hospital were included. Patients were stratified for age: 18-39 vs. 40-69 vs. ≥70 years (y). Aspartate aminotransferase (AST), alanine-aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT) and total bilirubin (BIL) were recorded.
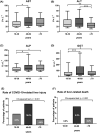
Results: 900 patients (18-39 years: 32.2%, 40-69 years: 39.7%, ≥70 years: 28.1%) were included. Number of comorbidities, median D-dimer and C-reactive protein increased with age. During COVID-19, AST/ALT and ALP/GGT levels significantly increased. Elevated hepatocellular transaminases (AST/ALT) and cholestasis parameters (ALP/GGT/BIL) were observed in 40.3% (n = 262/650) and 45.0% (n = 287/638) of patients respectively. Liver-related mortality was highest among patients with pre-existing decompensated liver disease (28.6%, p < .001). 1.7% of patients without pre-existing liver disease died of liver-related causes, that is consequences of hepatic dysfunction or acute liver failure. Importantly, COVID-19-associated liver injury (16.0%, p < .001), abnormal liver chemistries and liver-related mortality (6.5%, p < .001) were most frequent among 40-69 years old patients. Elevated AST and BIL after the first positive SARS-CoV-2 PCR independently predicted mortality in the overall cohort and in 40-69 years old patients.
Conclusions: Almost half of the COVID-19 patients exhibit abnormal hepatocellular and cholestasis-related liver chemistries with 40-69 years old patients being at particularly high risk for COVID-19-related liver injury and liver-related mortality. Elevated AST and BIL after SARS-CoV-2 infection are independent predictors of mortality, especially in patients aged 40-69 years.
Keywords: COVID-19; SARS-CoV-2; acute respiratory distress syndrome; liver chemistries; liver injury.
© 2022 The Authors. Liver International published by John Wiley & Sons Ltd.
Figures


References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8):782‐793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

