Changing Patterns of SARS-CoV-2 Seroprevalence among Canadian Blood Donors during the Vaccine Era
- PMID: 35412385
- PMCID: PMC9045380
- DOI: 10.1128/spectrum.00339-22
Changing Patterns of SARS-CoV-2 Seroprevalence among Canadian Blood Donors during the Vaccine Era
Abstract
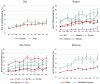
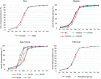
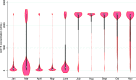
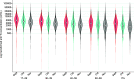
We monitored the seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid (anti-N; proxy of natural infection) and spike protein (anti-S; proxy for humoral immunity) antibodies in blood donors across Canada from January to November 2021. The first and second doses of vaccine were deployed over this time. Anti-N seroprevalence remained low overall (about 5% or lower) from January to November but was higher in racialized groups, younger age groups, and those living in materially deprived neighborhoods. Anti-S seroprevalence corresponded with the roll out of vaccines across the country, increasing in April in older donors and then progressively to younger age groups consistent with vaccination policies targeting oldest to youngest. By November, close to 100% of blood donors were positive for anti-S. Anti-S concentrations peaked by July and began waning by September to November particularly in older donors. These data have informed national and provincial public health policy in Canada throughout vaccination rollout. IMPORTANCE Throughout the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, our blood donor seroprevalence study has informed Canadian public health policy at national and provincial levels. We describe the only continuously running national seroprevalence study in Canada, which spans the full length of the pandemic and per capita is one of the largest programs in the world. The benefit of seroprevalence studies is that they identify a broad range of asymptomatic and symptomatic infection histories that may not be identified with active SARS-CoV-2 nucleic acid testing programs or when case definitions change. As vaccination was deployed in Canada, we estimated the proportion of donors with vaccine-related antibodies and developed population-level estimates of SARS-CoV-2 spike antibody concentrations. Monthly predictive mathematical models and our results engaged public health organizations in new ways. In the future, we intend to continue to expand on these interactions with provincial and national public health teams.
Keywords: Canada; SARS-CoV-2; blood donors; seroprevalence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cross-Canada Variability in Blood Donor SARS-CoV-2 Seroprevalence by Social Determinants of Health.Microbiol Spectr. 2023 Feb 14;11(1):e0335622. doi: 10.1128/spectrum.03356-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625634 Free PMC article.
-
Comparison of SARS-CoV-2 seroprevalence estimates between commercial lab serum specimens and blood donor specimens, United States, September-December 2021.Microbiol Spectr. 2024 Aug 6;12(8):e0012324. doi: 10.1128/spectrum.00123-24. Epub 2024 Jun 13. Microbiol Spectr. 2024. PMID: 38869287 Free PMC article.
-
SARS-CoV-2 Virus-Like Particle Neutralizing Capacity in Blood Donors Depends on Serological Profile and Donor-Declared SARS-CoV-2 Vaccination History.Microbiol Spectr. 2022 Feb 23;10(1):e0226221. doi: 10.1128/spectrum.02262-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171006 Free PMC article.
-
Seroprevalence of SARS-CoV-2 antibodies among healthy blood donors: a systematic review and meta-analysis.BMC Public Health. 2024 Oct 22;24(1):2925. doi: 10.1186/s12889-024-20364-5. BMC Public Health. 2024. PMID: 39438911 Free PMC article.
-
Current challenges of severe acute respiratory syndrome coronavirus 2 seroprevalence studies among blood donors: A scoping review.Vox Sang. 2022 Apr;117(4):476-487. doi: 10.1111/vox.13221. Epub 2021 Dec 3. Vox Sang. 2022. PMID: 34862614
Cited by
-
Cross-Canada Variability in Blood Donor SARS-CoV-2 Seroprevalence by Social Determinants of Health.Microbiol Spectr. 2023 Feb 14;11(1):e0335622. doi: 10.1128/spectrum.03356-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625634 Free PMC article.
-
Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022.J Korean Med Sci. 2024 Feb 5;39(5):e43. doi: 10.3346/jkms.2024.39.e43. J Korean Med Sci. 2024. PMID: 38317445 Free PMC article.
-
SARS-CoV-2 vaccination in Canadian blood donors: Insight into donor representativeness of the general population.Vaccine X. 2024 May 12;18:100498. doi: 10.1016/j.jvacx.2024.100498. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38800670 Free PMC article.
-
Influenza and COVID-19 vaccination in Canadian blood donors: A comparison across pre- and post-pandemic periods.Vox Sang. 2025 May;120(5):464-472. doi: 10.1111/vox.70006. Epub 2025 Feb 27. Vox Sang. 2025. PMID: 40015315 Free PMC article.
-
SARS-CoV-2 Seroprevalence among Canadian Blood Donors: The Advance of Omicron.Viruses. 2022 Oct 25;14(11):2336. doi: 10.3390/v14112336. Viruses. 2022. PMID: 36366432 Free PMC article.
References
-
- Public Health Agency of Canada (PHAC). 2021. Coronavirus disease (COVID-19): outbreak update. https://canada.ca/en/public-health/services/diseases/2019-novel-coronavi.... Accessed January 18, 2022.
-
- Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, Di Germanio C, Green V, Notari E, Saa P, Biggerstaff BJ, Strauss D, Kessler D, Vassallo R, Reik R, Rossmann S, Destree M, Nguyen K-A, Sayers M, Lough C, Bougie DW, Ritter M, Latoni G, Weales B, Sime S, Gorlin J, Brown NE, Gould CV, Berney K, Benoit TJ, Miller MJ, Freeman D, Kartik D, Fry AM, Azziz-Baumgartner E, Hall AJ, MacNeil A, Gundlapalli AV, Basavaraju SV, Gerber SI, Patton ME, Custer B, Williamson P, Simmons G, Thornburg NJ, Kleinman S, Stramer SL, Opsomer J, Busch MP. 2021. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA 326:1400–1409. doi:10.1001/jama.2021.15161. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

