Efficacy of a novel large-cell Niti-S stent with a slim delivery system for hilar biliary obstruction: a preliminary study
- PMID: 35412414
- PMCID: PMC9009962
- DOI: 10.1080/07853890.2022.2056631
Efficacy of a novel large-cell Niti-S stent with a slim delivery system for hilar biliary obstruction: a preliminary study
Abstract
Objectives: The large-cell Niti-S stent is useful for multiple stenting in patients with malignant hilar biliary obstruction (MHBO). Recently, a novel uncovered self-expandable metallic stent (USEMS) (a Niti-S large-cell SR slim delivery system) was developed. In this study, we aimed to evaluate the efficacy of this USEMS slim delivery system in MHBO patients.
Materials and methods: Outcomes related to USEMS placement, the clinical course, and the period to recurrent biliary obstruction (RBO) were evaluated in MHBO patients who received multiple USEMSs with the Niti-S large-cell SR slim delivery system.
Results: Twenty-two MHBO patients underwent the placement of multiple USEMSs, including the novel slim-delivery stent. Six patients had a past history of upper gastrointestinal reconstruction (Billroth I: 1, Billroth II: 4, Roux-en-Y: 1). The number of USEMSs placed in each patient was 2-6. Three procedures were reinterventions. The new slim delivery system was placed as the first stent in ten patients and as an additional stent in the remaining patients. Seven patients were drained using only Niti-S large-cell SR slim delivery stents. The technical and clinical success rates were both 100%.
Conclusions: Placing multiple USEMSs in patients with a past history of abdominal surgery or in reintervention is difficult. Although difficult cases were included in this study, stent-in-stent placement with the novel Niti-S large-cell SR slim delivery system was useful in treating MHBO patients. In addition, this novel stent might be the first choice for MHBO patients.KEY MESSAGESEndoscopic multistenting for MHBO is challenging. In addition, reintervention or multistenting for MHBO patients with a past history of abdominal surgery becomes more difficult.The novel Niti-S large-cell SR slim delivery USEMS is useful as an additional stent because the delivery system is thin and suitable for a 0.025 guidewire. In addition, the novel stent is of the braided type and has a large mesh. Therefore, the novel stent is expected to have strong radial force and can be used as the first SEMS.The Niti-S large-cell SR slim delivery stent is long enough to be used in patients with upper gastrointestinal reconstruction. Although this study included patients with reintervention or a past history of upper gastrointestinal reconstruction, the technical success rate of multiple stenting for MHBO patients was 100%. The slim-delivery stent might overcome several difficulties of endoscopic multistenting.
Keywords: Large-cell stent; malignant hilar biliary obstruction; slim delivery.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

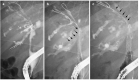
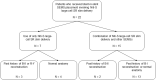
References
-
- Mukai T, Yasuda I, Nakashima M, et al. . Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013:214–222. - PubMed
-
- Xia MX, Pan YL, Cai XB, et al. . Comparison of endoscopic bilateral metal stent drainage with plastic stents in the palliation of unresectable hilar biliary malignant strictures: large multicenter study. Dig Endosc. 2021;33(1):179–189. - PubMed
-
- Lee TH, Kim TH, Moon JH, et al. . Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video). Gastrointest Endosc. 2017;86(5):817–827. - PubMed
-
- Teng F, Xian YT, Lin J, et al. . Comparison of unilateral with bilateral metal stenting for malignant hilar biliary obstruction. Surg Laparosc Endosc Percutan Tech. 2019;29(1):43–48. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
