Rasch-calibrated Intermittent Exotropia Symptom Questionnaire for Children
- PMID: 35412528
- PMCID: PMC9249164
- DOI: 10.1097/OPX.0000000000001901
Rasch-calibrated Intermittent Exotropia Symptom Questionnaire for Children
Abstract
Significance: A rigorously designed and calibrated symptom questionnaire for childhood intermittent exotropia would be useful for clinical care and for research.
Purpose: The aim of this study was to Rasch-calibrate and evaluate the previously developed Child Intermittent Exotropia Symptom Questionnaire using data gathered as part of a randomized clinical trial.
Methods: The questionnaire was administered to 386 children aged 3 to 10 years with intermittent exotropia who were enrolled in a randomized clinical trial comparing overminus with nonoverminus spectacles. Participants were followed at 6 and 12 months while on treatment and at 18 months off treatment. Factor analysis determined dimensionality, and Rasch analysis evaluated questionnaire performance. Logit values were converted to 0 (best) to 100 (worst). We evaluated differences in questionnaire scores between treatment groups and time points, and correlations with control scores.
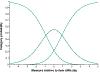
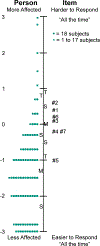
Results: The Child Intermittent Exotropia Symptom Questionnaire was unidimensional. Rasch analysis indicated that there was no notable local dependence and no significant differential item functioning for sex or age. There was suboptimal targeting (mean logit, -1.62), and person separation was somewhat poor (0.95). There were no significant differences in the Child Intermittent Exotropia Symptom score between overminus spectacles and nonoverminus spectacles at 6, 12, and 18 months. Combining data from both treatment groups, there was significant improvement from baseline at all follow-up visits (e.g., mean change from baseline to 12 months, -6.6 points; 95% confidence interval, -8.6 to -4.6). Child Intermittent Exotropia Symptom scores were not correlated with distance or near control scores at 12 months.
Conclusions: The seven-item Rasch-scored Child Intermittent Exotropia Symptom Questionnaire is limited by suboptimal performance. Future study is needed to determine whether it may be useful for clinical practice and for research.
Trial registration: ClinicalTrials.gov NCT02807350.
Copyright © 2022 American Academy of Optometry.
Conflict of interest statement
Conflict of Interest Disclosure: None of the authors have reported a financial conflict of interest.
Figures



References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

