Effect of Antimicrobial Prophylaxis Duration on Health Care-Associated Infections After Clean Orthopedic Surgery: A Cluster Randomized Trial
- PMID: 35412627
- PMCID: PMC9006110
- DOI: 10.1001/jamanetworkopen.2022.6095
Effect of Antimicrobial Prophylaxis Duration on Health Care-Associated Infections After Clean Orthopedic Surgery: A Cluster Randomized Trial
Abstract
Importance: Postoperative health care-associated infections are associated with a greater deterioration in patients' general health status and social and economic burden, with at least 1 occurring in approximately 4% of acute care hospital patients. Antimicrobial prophylaxis prevents surgical site infections in various orthopedic procedures; however, its relationship with health care-associated infections remains unknown.
Objective: To examine whether a shorter antimicrobial prophylaxis duration of less than 24 hours after surgery is not inferior to a longer duration in preventing health care-associated infections after clean orthopedic surgery.
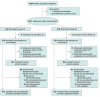
Design, setting, and participants: This open-label, multicenter, cluster randomized, noninferiority clinical trial was conducted in 5 tertiary referral hospitals in greater Tokyo metropolitan area, Japan, from May to December 2018. Adult patients undergoing clean orthopedic surgery were recruited until the planned number of participants was achieved (500 participants per group). Statistical analysis was conducted from July to December 2019.
Interventions: Antimicrobial prophylaxis was discontinued within 24 hours after surgery in group 24 and 24 to 48 hours after surgery in group 48. Group allocation was switched every 2 or 4 months according to the facility-based cluster rule. Study-group assignments were masked from participants.
Main outcomes and measures: The primary outcome was the incidence of health care-associated infections requiring antibiotic therapies within 30 days after surgery. The noninferiority margin was 4%.
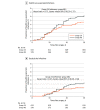
Results: Of the 1211 participants who underwent cluster allocation, 633 participants were in group 24 (median [IQR] age, 73 [61-80] years; 250 men [39.5%] and 383 women [60.5%]), 578 participants were in group 48 (median [IQR] age, 74 [62-81] years; 204 men [35.3%] and 374 women [64.7%]), and all were eligible for the intention-to-treat analyses. Health care-associated infections occurred in 29 patients (4.6%) in group 24 and 38 patients (6.6%) in group 48. Intention-to-treat analyses showed a risk difference of -1.99 percentage points (95% CI, -5.05 to 1.06 percentage points; P < .001 for noninferiority) between groups, indicating noninferiority. Results of adjusted intention-to-treat, per-protocol, and per designated procedure population analyses supported this result, without a risk of antibiotic resistance and prolonged hospitalization.
Conclusions and relevance: This cluster randomized trial found noninferiority of a shorter antimicrobial prophylaxis duration in preventing health care-associated infections without an increase in antibiotic resistance risk. These findings lend support to the global movement against antimicrobial resistance and provide additional information on adequate antimicrobial prophylaxis for clean orthopedic surgery.
Trial registration: Identifier: UMIN000030929.
Conflict of interest statement
Figures

References
-
- Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198-1208. doi:10.1056/NEJMoa1306801 - DOI - PMC - PubMed
-
- World Health Organization . Global Guidelines for the Prevention of Surgical Site Infection. 2016. Accessed October 1, 2020. https://apps.who.int/iris/bitstream/handle/10665/250680/9789241549882-en... - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions

