Nerve growth factor transfer from cardiomyocytes to innervating sympathetic neurons activates TrkA receptors at the neuro-cardiac junction
- PMID: 35413134
- PMCID: PMC9321700
- DOI: 10.1113/JP282828
Nerve growth factor transfer from cardiomyocytes to innervating sympathetic neurons activates TrkA receptors at the neuro-cardiac junction
Abstract
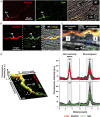
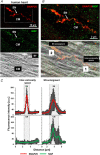
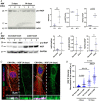
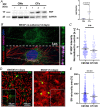
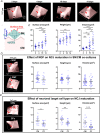
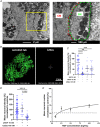
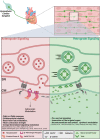
Sympathetic neurons densely innervate the myocardium with non-random topology and establish structured contacts (i.e. neuro-cardiac junctions, NCJ) with cardiomyocytes, allowing synaptic intercellular communication. Establishment of heart innervation is regulated by molecular mediators released by myocardial cells. The mechanisms underlying maintenance of cardiac innervation in the fully developed heart, are, however, less clear. Notably, several cardiac diseases, primarily affecting cardiomyocytes, are associated with sympathetic denervation, supporting the hypothesis that retrograde 'cardiomyocyte-to-sympathetic neuron' communication is essential for heart cellular homeostasis. We aimed to determine whether cardiomyocytes provide nerve growth factor (NGF) to sympathetic neurons, and the role of the NCJ in supporting such retrograde neurotrophic signalling. Immunofluorescence on murine and human heart slices shows that NGF and its receptor, tropomyosin-receptor-kinase-A, accumulate, respectively, in the pre- and post-junctional sides of the NCJ. Confocal immunofluorescence, scanning ion conductance microscopy and molecular analyses, in co-cultures, demonstrate that cardiomyocytes feed NGF to sympathetic neurons, and that this mechanism requires a stable intercellular contact at the NCJ. Consistently, cardiac fibroblasts, devoid of NCJ, are unable to sustain SN viability. ELISA assay and competition binding experiments suggest that this depends on the NCJ being an insulated microenvironment, characterized by high [NGF]. In further support, real-time imaging of tropomyosin-receptor-kinase-A vesicle movements demonstrate that efficiency of neurotrophic signalling parallels the maturation of such structured intercellular contacts. Altogether, our results demonstrate the mechanisms which link sympathetic neuron survival to neurotrophin release by directly innervated cardiomyocytes, conceptualizing sympathetic neurons as cardiomyocyte-driven heart drivers. KEY POINTS: CMs are the cell source of nerve growth factor (NGF), required to sustain innervating cardiac SNs; NCJ is the place of the intimate liaison, between SNs and CMs, allowing on the one hand neurons to peremptorily control CM activity, and on the other, CMs to adequately sustain the contacting, ever-changing, neuronal actuators; alterations in NCJ integrity may compromise the efficiency of 'CM-to-SN' signalling, thus representing a potentially novel mechanism of sympathetic denervation in cardiac diseases.
Keywords: cardiac sympathetic neurons; cardiomyocytes; nerve growth factor; nerve growth factor receptor; neuro-cardiac junction.
© 2022 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures



Comment in
-
Bridging the communication gap: cardiomyocytes reciprocate sympathetic nerve signalling.J Physiol. 2022 Jun;600(12):2827-2828. doi: 10.1113/JP283173. Epub 2022 May 25. J Physiol. 2022. PMID: 35614020 Free PMC article. No abstract available.
References
-
- Berg, M. M. , Sternberg, D. W. , Parada, L. F. , & Chao, M. V. (1992). K‐252a inhibits nerve growth factor‐induced trk proto‐oncogene tyrosine phosphorylation and kinase activity. Journal of Biological Chemistry, 267, 13–16. - PubMed
-
- Di Bona, A. , Vita, V. , Costantini, I. , & Zaglia, T. (2020). Towards a clearer view of sympathetic innervation of cardiac and skeletal muscles. Progress in Biophysics and Molecular Biology, 154, 80–93. - PubMed
-
- Boogers, M. J. , Borleffs, C. J. W. , Henneman, M. M. , van Bommel, R. J. , van Ramshorst, J. , Boersma, E. , Dibbets‐Schneider, P. , Stokkel, M. P. , van der Wall, E. E. , Schalij, M. J. , & Bax, J. J. (2010). Cardiac sympathetic denervation assessed with 123‐iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter‐defibrillator patients. Journal of the American College of Cardiology, 55, 2769–2777. - PubMed
-
- Cao, J.‐M. , Chen, L. S. , KenKnight, B. H. , Ohara, T. , Lee, M.‐H. , Tsai, J. , Lai, W. W. , Karagueuzian, H. S. , Wolf, P. L. , Fishbein, M. C. , & Chen, P.‐S. (2000). Nerve sprouting and sudden cardiac death. Circulation Research, 86, 816–821. - PubMed
-
- Cao, J.‐M. , Fishbein, M. C. , Han, J. B. , Lai, W. W. , Lai, A. C. , Wu, T.‐J. , Czer, L. , Wolf, P. L. , Denton, T. A. , Shintaku, I. P. , Chen, P.‐S. , & Chen, L. S. (2000). Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation, 101, 1960–1969. - PubMed

