Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies
- PMID: 35414025
- PMCID: PMC9006445
- DOI: 10.1186/s13045-022-01260-0
Targeting regulated cell death (RCD) with small-molecule compounds in triple-negative breast cancer: a revisited perspective from molecular mechanisms to targeted therapies
Abstract
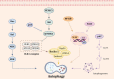
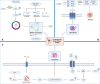
Triple-negative breast cancer (TNBC) is a subtype of human breast cancer with one of the worst prognoses, with no targeted therapeutic strategies currently available. Regulated cell death (RCD), also known as programmed cell death (PCD), has been widely reported to have numerous links to the progression and therapy of many types of human cancer. Of note, RCD can be divided into numerous different subroutines, including autophagy-dependent cell death, apoptosis, mitotic catastrophe, necroptosis, ferroptosis, pyroptosis and anoikis. More recently, targeting the subroutines of RCD with small-molecule compounds has been emerging as a promising therapeutic strategy, which has rapidly progressed in the treatment of TNBC. Therefore, in this review, we focus on summarizing the molecular mechanisms of the above-mentioned seven major RCD subroutines related to TNBC and the latest progress of small-molecule compounds targeting different RCD subroutines. Moreover, we further discuss the combined strategies of one drug (e.g., narciclasine) or more drugs (e.g., torin-1 combined with chloroquine) to achieve the therapeutic potential on TNBC by regulating RCD subroutines. More importantly, we demonstrate several small-molecule compounds (e.g., ONC201 and NCT03733119) by targeting the subroutines of RCD in TNBC clinical trials. Taken together, these findings will provide a clue on illuminating more actionable low-hanging-fruit druggable targets and candidate small-molecule drugs for potential RCD-related TNBC therapies.
Keywords: Anoikis; Apoptosis; Autophagy-dependent cell death; Combination strategy; Ferroptosis; Mitotic catastrophe; Necroptosis; Pyroptosis; Regulated cell death (RCD); Triple-negative breast cancer (TNBC).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Islam S, Dasgupta H, Basu M, Roy A, Alam N, Roychoudhury S, et al. Downregulation of beta-catenin in chemo-tolerant TNBC through changes in receptor and antagonist profiles of the WNT pathway: clinical and prognostic implications. Cell Oncol (Dordrecht) 2020;43(4):725–741. doi: 10.1007/s13402-020-00525-5. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

