Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial
- PMID: 35414591
- PMCID: PMC9006844
- DOI: 10.1136/jitc-2021-004424
Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial
Abstract
Background: Availability of checkpoint inhibitors has created a paradigm shift in the management of patients with solid tumors. Despite this, most patients do not respond to immunotherapy, and there is considerable interest in developing combination therapies to improve response rates and outcomes. B7-H3 (CD276) is a member of the B7 family of cell surface molecules and provides an alternative immune checkpoint molecule to therapeutically target alone or in combination with programmed cell death-1 (PD-1)-targeted therapies. Enoblituzumab, an investigational anti-B7-H3 humanized monoclonal antibody, incorporates an immunoglobulin G1 fragment crystallizable (Fc) domain that enhances Fcγ receptor-mediated antibody-dependent cellular cytotoxicity. Coordinated engagement of innate and adaptive immunity by targeting distinct members of the B7 family (B7-H3 and PD-1) is hypothesized to provide greater antitumor activity than either agent alone.
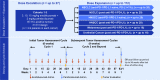
Methods: In this phase I/II study, patients received intravenous enoblituzumab (3-15 mg/kg) weekly plus intravenous pembrolizumab (2 mg/kg) every 3 weeks during dose-escalation and cohort expansion. Expansion cohorts included non-small cell lung cancer (NSCLC; checkpoint inhibitor [CPI]-naïve and post-CPI, programmed death-ligand 1 [PD-L1] <1%), head and neck squamous cell carcinoma (HNSCC; CPI-naïve), urothelial cancer (post-CPI), and melanoma (post-CPI). Disease was assessed using Response Evaluation Criteria in Solid Tumors version 1.1 after 6 weeks and every 9 weeks thereafter. Safety and pharmacokinetic data were provided for all enrolled patients; efficacy data focused on HNSCC and NSCLC cohorts.
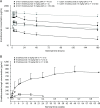
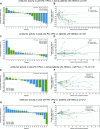
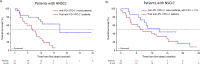
Results: Overall, 133 patients were enrolled and received ≥1 dose of study treatment. The maximum tolerated dose of enoblituzumab with pembrolizumab at 2 mg/kg was not reached. Intravenous enoblituzumab (15 mg/kg) every 3 weeks plus pembrolizumab (2 mg/kg) every 3 weeks was recommended for phase II evaluation. Treatment-related adverse events occurred in 116 patients (87.2%) and were grade ≥3 in 28.6%. One treatment-related death occurred (pneumonitis). Objective responses occurred in 6 of 18 (33.3% [95% CI 13.3 to 59.0]) patients with CPI-naïve HNSCC and in 5 of 14 (35.7% [95% CI 12.8 to 64.9]) patients with CPI-naïve NSCLC.
Conclusions: Checkpoint targeting with enoblituzumab and pembrolizumab demonstrated acceptable safety and antitumor activity in patients with CPI-naïve HNSCC and NSCLC.
Trial registration number: NCT02475213.
Keywords: clinical trials as topic; drug therapy, combination; head and neck neoplasms; immunotherapy; lung neoplasms.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: CA reports personal fees from AstraZeneca, BluPrint Oncology, Celgene, Eli Lilly, Merck, Daiichi Sankyo for advisory boards, and research funding to the institution from Merck, MacroGenics, Novartis, and AstraZeneca; SA reports consulting fees from Bristol-Myers Squibb, Merck, CBMG, AstraZeneca, Memgen, RAPT, Venn, Achilles Therapeutics, Celsius, Samyang Biopharma, GlaxoSmithKline, Amgen, travel expenses from Bristol-Myers Squibb, Merck, Rapt, Achilles Therapeutics, Celsius, GlaxoSmithKline, Amgen, and grants from Novartis; VA reports personal fees and non-financial support from Bristol-Myers Squibb, and personal fees from Merck, MSD, Novartis, QBiotics, Nektar, Roche, Pierre Fabre; RC reports grants paid to the institution from MacroGenics, Merck, personal fees from AstraZeneca, grants to the institution and personal fees from Innate Pharma, Genocea Biosciences, Heat Biologics, advisory boards for Innate Pharma, Genocea Biosciences, AstraZeneca, Heat Biologics, Cantargia, research funding from Celldex, Kyn Therapeutics, Merck, Genocea Biosciences, Xencor, Innate Pharma, Heat Biologics, and travel expenses from Heat Biologics, Innate Pharma, Genocea Biosciences; RLF reports consulting fees from Aduro Biotech, Novasenta, Sanofi, Zymeworks, honoraria from Bristol-Myers Squibb, EMD Serono, MacroGenics, Merck, Numab Therapeutics, Pfizer for advisory boards, research funds to institution from AstraZeneca/MedImmune, Bristol-Myers Squibb, Novasenta, Tesaro, clinical trial support from AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck, and stocks in Novasenta; VG reports honoraria from AstraZeneca, Roche/Genentech; ARK reports holding stocks in ECOM Medical, receiving consulting fees from Exelixis, AstraZeneca, Bayer, Pfizer, Novartis, Genentech, Bristol-Myers Squibb, EMD Serono, serving on Speakers’ Bureau at Janssen, Astellas Medivation, Pfizer, Novartis, Sanofi, Genentech/Roche, Eisai, AstraZeneca, Bristol-Myers Squibb, Amgen, Exelixis, EMD Serono, Merck, Seattle Genetics/Astellas, receiving research funding to the institution from Genentech, Exelixis, Janssen, AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, MacroGenics, Astellas Pharma, beyond spring, BioClin Therapeutics, Clovis Oncology, Bavarian Nordic, Seattle Genetics, Immunomedics, Epizyme, and receiving travel expenses from Genentech, Prometheus Laboratories, Astellas Medivation, Janssen, Eisai, Bayer, Pfizer, Novartis, Exelixis, AstraZeneca; SK reports consulting fees from MacroGenics and is a stock holder of Abbott, Aqua, Bank of America, Baxter, Betterment, Bristol-Myers Squibb, Etrade, ExxonMobil, Invesco, Johnson & Johnson, Lockheed Martin Corp, Marathon Oil, Marathon Petroleum, Pfizer, Takeda Pharm Co, Verizon, Welltower, Vanguard; NL reports research funding from ALX Therapeutics, Ascentage, Asana, BeiGene, Constellation Pharma, Alexion, Cerulean, Forty Seven, Alpine, Merck, Pfizer, Regeneron, TaiRx, Apexian, Formation Biologics (Forbius), Symphogen, CytomX, InhibRx, Incyte, Jounce, Livzon, Northern Biologics, Innovent Biologics, Ikena, Odonate, Loxo, Alpine Biosciences, Ikena, Mersana, and serves on advisory boards for Innovent Biologics; YL reports research funding from Merck, MacroGenics, Tolero Pharmaceuticals, AstraZeneca, Vaccinex, Blueprint Medicines, Harpoon Therapeutics, Sun Pharma Advanced Research, Kyowa Pharmaceuticals, Tesaro, Bayer HealthCare, honorarium from Clarion Healthcare, served on the advisory board for AstraZeneca, Novocure, and consultant for AstraZeneca; OR reports research support from Merck, Speakers’ Bureau activities supported by educational grants from Bristol-Myers Squibb and Merck, and honoraria for consulting activities for Merck, Celgene, Five Prime, GlaxoSmithKline, Bayer, Roche/Genentech, Puretech, Imvax, Sobi. In addition, OR has a patent pending for 'Methods of using pembrolizumab and trebananib'; MS received honoraria from Deciphera Pharmaceuticals and Daiichi Sankyo for serving on advisory boards; AT reports consulting fees from AbbVie, Adagene, Agenus, Aro Biotherapeutics, Ascentage, Aximmune, Bayer, BioInvent, Birdie, Cello Health, Ellipses, EMD Serono, Five Prime, Forbius, Gilde Healthcare Partners, HBM Partners, Immunomet, Karma Oncology, Mekanistic, Menarini, Mersana, Nanobiotix, Nuvalent, OSI, Partner Therapeutics, Pfizer, Pieris, Pierre Fabre, Ridgeway, RYVU Therapeutics, Scitemex, Seattle Genetics, Symphogen, Syneos, TFS Trial Form Support, and Trillium Therapeutics, and honoraria from Abgenomics, Adagene, ADC Therapeutics, Aro Biotherapeutics, BioInvent, Elucida, Immunome, Jazz, NBE Therapeutics, Pelican, Innate Pharma, TFS Trial Form Support International, and Zymeworks for serving on advisory boards. In addition, AT serves on the Independent Data Monitoring Committees for Mirati and Genentech; NV reports non-financial support for conducting trials with Amgen, Aravive, Arrowhead, Arvinas, AstraZeneca, Bayer, Bristol-Myers Squibb, Calithera, Clovis, Corvus, Dendreon, eFFECTOR, Eisai, Endocyte, Epizyme, ESSA, Exelixis, Genentech, Immunomedics, Kangpu, Kinter Suzhou, MacroGenics, Merck, Mirati, Nektar, Novartis, Seattle Genetics, Tolero, advisory boards for Amgen, Astellas, AstraZeneca, Aveo, Caris, Clovis, Corvus, Eisai, Exelixis, GeneCentric, Genentech, Janssen, Merck, Myovant, OnQuality Pharma, Pfizer, Sanofi-Genzyme, Tolero, serves on the Speakers’ Bureau at Bayer, Caris, Clovis, Sanofi-Genzyme, Seattle Genetics, consulting services to Cancer Expert Now, vice-chair responsibilities at SWOG, section editor at Up-To-Date, and legal defense advisor to Novartis, Sanofi-Genzyme; AW reports grants from Merck, grants and personal fees from Bristol-Myers Squibb, personal fees from Ipsen, Pfizer; DPZ received institutional research support from Merck, Bristol-Myers Squibb, GlaxoSmithKline, AstraZeneca, Aduro Biotech, Lilly, Astellas, MacroGenics for his role as a PI of clinical trials sponsored by these companies and received honoraria from Blueprint Medicines for serving on an advisory board; SMG is a former employee of MacroGenics serving as medical monitor; XZ, RC, and LP are employees of MacroGenics; JB was an employee of MacroGenics; PAM is an employee of MacroGenics, holds stock in MacroGenics, and holds a patent with all rights assigned to MacroGenics for 'Antibodies reactive with B7-H3, immunologically active fragments thereof' US Patent 9,150,656; EB is an employee of MacroGenics, holds stock in MacroGenics, and has a patent US20190389952 pending to MacroGenics; FA was an employee of MacroGenics and is now an employee of AstraZeneca; AP, AAA, AB, RH, and AMJ have nothing to disclose.
Figures

References
-
- Shitara K, Van Cutsem E, Bang Y-J, et al. . Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 2020;6:1571–80. 10.1001/jamaoncol.2020.3370 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
