CD157 Can Replace CD24 and CD14 in a Single-Tube Flow-Cytometric Assay to Detect Paroxysmal Nocturnal Hemoglobinuria (PNH) Clones on Both Neutrophils and Monocytes: A Prospective Study From North India
- PMID: 35415060
- PMCID: PMC8994018
- DOI: 10.7759/cureus.23965
CD157 Can Replace CD24 and CD14 in a Single-Tube Flow-Cytometric Assay to Detect Paroxysmal Nocturnal Hemoglobinuria (PNH) Clones on Both Neutrophils and Monocytes: A Prospective Study From North India
Abstract
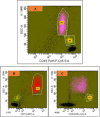
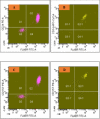
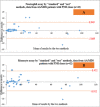
Introduction As per current guidelines, detection of paroxysmal nocturnal hematuria (PNH) clones on leucocytes requires the demonstration of the loss of at least two glycosyl-phosphatidyl-inositol (GPI)-linked molecules on both neutrophils and monocytes by flow cytometry. CD24 and CD14 are GPI-linked molecules expressed on neutrophils and monocytes respectively, whereas another GPI-linked molecule, CD157, is expressed on both neutrophils and monocytes. This prospective study evaluated the ability of CD157 to replace both CD24 and CD14 in a single-tube flow-cytometric assay to detect PNH clones on both neutrophils and monocytes. Materials and methods PNH clones were newly detected in 52 patients by an existing "standard" single-tube six-color flow-cytometric method, which was routinely performed in our laboratory at the time of undertaking this study. Six antibodies (CD45/CD15/CD64/CD24/CD14/FLAER) were used in this "standard" technique. Subjects were divided into two groups: (i) PNH disease (n=10), and (ii) aplastic anemia/myelodysplastic syndrome (AA/MDS) (n=42). Diagnosis of PNH disease and AA/MDS were made as per standard literature and guidelines. Results were compared with a single-tube five-color "test" assay using the antibodies CD45/CD15/CD64/CD157/FLAER by flow cytometry. Samples from 20 healthy control subjects were used to calculate cut-off values for the "test" assay. Results By the "test" method, cut-off values for detecting PNH clones obtained from receiver operating-characteristic curve analysis were >0.4% for neutrophils (sensitivity=96.15%, specificity=95%), and >0.9% for monocytes (sensitivity=98.08%, specificity=95%). There was significant correlation between PNH clone sizes measured by both the "standard" and "test" assays in neutrophils (PNH disease: r=0.976, p<0.001; AA/MDS: r=0.980, p<0.001) as well as monocytes (PNH disease: r=0.806, p=0.005; AA/MDS: r=0.915, p<0.001). Bland-Altman analysis showed agreement between both assays in all the 52 patients and in individuals with AA/MDS. The cost of the test to the patients was about 15% less in the "test" method than the "standard" technique, with improved technical efficiency. Conclusion CD157 can replace both CD24 and CD14 in a single-tube flow-cytometric assay to detect PNH clones on both neutrophils and monocytes, with reduced cost to the patients and improved technical efficiency.
Keywords: cd14; cd157; cd24; flaer; flow cytometry; paroxysmal nocturnal hemoglobinuria; pnh.
Copyright © 2022, Deka et al.
Conflict of interest statement
The first author hereby declares that although there are no personal/family relationships with any of the five author-suggested reviewers, three of them were his ex-colleagues at the department where this study was carried out, and one of them is presently working with him at his current institute as a clinical hematologist.
Figures



References
-
- Parker CJ, Ware RE. Wintrobe’s Clinical Hematology, 14th ed. Philadelphia, PA: Wolters Kluwer; 2019. Paroxysmal nocturnal hemoglobinuria; pp. 783–810.
-
- Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Borowitz MJ, Craig FE, Digiuseppe JA, et al. Cytometry B Clin Cytom. 2010;78:211–230. - PubMed
-
- Practical guidelines for the high-sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by flow cytometry. Sutherland DR, Keeney M, Illingworth A. Cytometry B Clin Cytom. 2012;82:195–208. - PubMed
-
- ICCS/ESCCA consensus guidelines to detect GPI-deficient cells in paroxysmal nocturnal hemoglobinuria (PNH) and related disorders part 3 - data analysis, reporting and case studies. Illingworth A, Marinov I, Sutherland DR, Wagner-Ballon O, DelVecchio L. Cytometry B Clin Cytom. 2018;94:49–66. - PubMed
-
- Use of CD157 in FLAER-based assays for high-sensitivity PNH granulocyte and PNH monocyte detection. Sutherland DR, Acton E, Keeney M, Davis BH, Illingworth A. Cytometry B Clin Cytom. 2014;86:44–55. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
