Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
- PMID: 35415183
- PMCID: PMC8928186
- DOI: 10.1159/000518574
Use of Botulinum Toxin for Androgenic Alopecia: A Systematic Review
Erratum in
-
Erratum.Skin Appendage Disord. 2022 Mar;8(2):101. doi: 10.1159/000521354. Epub 2021 Dec 22. Skin Appendage Disord. 2022. PMID: 35420758 Free PMC article.
Abstract
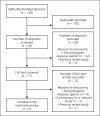
In this systematic review, we summarize the efficacy and safety of intradermal and intramuscular botulinum toxin injections for androgenic alopecia (AGA). Using PubMed, we conducted a literature search up to February 2021 using the following keyword combinations: "botulinum toxin" or "botox" and "androgenetic alopecia," "hair loss," or "alopecia." Five clinical studies met our inclusion criteria: 4 prospective cohorts and 1 randomized clinical trial (RCT). Study durations ranged from 24 to 60 weeks. No studies included control groups or compared botulinum toxin injections against approved treatments. A total of 165 participants were identified - all of whom were males with AGA. Of the 4 studies measuring response rates (i.e., subjects with >0% hair changes), response rates ranged from 75 to 79.1%. Within studies measuring hair count changes from intramuscular injections, changes ranged from 18 to 20.9%. No serious adverse events were reported. Studies on botulinum toxin injections have produced favorable outcomes for AGA subjects. However, results should be interpreted with caution due to the absence of control groups, small numbers of participants, and relatively low Jadad quality scores. Large RCTs are recommended to confirm efficacy and safety, explore the effects of botulinum toxin on females with pattern hair loss, and establish best practices for intradermal and intramuscular injection methodologies.
Keywords: Androgenic alopecia; Botulinum toxin; Hair loss.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
Robert English and Sophia Ruiz have no conflicts of interest to declare.
Figures
References
-
- Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A, et al. Androgenetic alopecia: a review. Endocrine. 2017 Jul;57((1)):9–17. - PubMed
-
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002 Dec;16((14)):1967–9. - PubMed
-
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Identification of androgen-inducible TGF-beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Investig Dermatol Symp Proc. 2003 Jun;8((1)):69–71. - PubMed
-
- Winiarska A, Mandt N, Kamp H, Hossini A, Seltmann H, Zouboulis CC, et al. Effect of 5alpha-dihydrotestosterone and testosterone on apoptosis in human dermal papilla cells. Skin Pharmacol Physiol. 2006;19((6)):311–21. - PubMed
-
- Kaufman KD, Dawber RP. Finasteride, a Type 2 5alpha-reductase inhibitor, in the treatment of men with androgenetic alopecia. Expert Opin Investig Drugs. 1999 Apr;8((4)):403–15. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous