Drug-induced corneal deposits: an up-to-date review
- PMID: 35415268
- PMCID: PMC8961126
- DOI: 10.1136/bmjophth-2021-000943
Drug-induced corneal deposits: an up-to-date review
Abstract
This review assesses different clinical aspects of the various known drug-induced corneal deposits, based on the corneal layer involved (epithelium, stroma and/or endothelium), and based on the drug class. The most well-known condition caused by drug deposits is vortex keratopathy, or corneal verticillata, which is a whorl-like opacity in the corneal epithelium. Vortex keratopathy is commonly caused by certain cationic amphiphilic drugs such as amiodarone, antimalarials, suramin, tamoxifen, chlorpromazine and non-steroidal anti-inflammatory drugs. These deposits usually occur once a certain dose of the drug is reached. Most cases present with mild to moderate symptoms with minimal visual impairment. Most of these deposits resolve automatically, after months to years of drug cessation. Notably, other drug classes can cause deposits in all three layers of the cornea. Chlorpromazine, gold, rifabutin, indomethacin and tyrosine kinase inhibitors can cause stromal deposits, with reduced visual acuity when the anterior stroma is involved. Chlorpromazine and rifabutin can also cause deposits in the endothelial layer of the cornea. Regardless of the type of corneal deposit, local therapies such as topical lubricants or corticosteroids may help improve symptoms. Drug cessation or modification can also be helpful but should be weighed against the systemic risks of the underlying disease.
Keywords: cornea; drugs; ocular surface.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None of the authors has any financial/competing interests to disclose, except M-CR who discloses being a consultant for TALLC, Han-ALL, Santen and an employee of EBSCO. This study does not contain any personal identifying information.
Figures

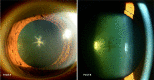
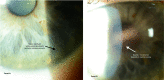
References
-
- Weiss JS, Moller H, Aldave A. IC3D classification of corneal dystrophies. Edition 2. Cornea, 2015: 117–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
