Sequential gastrodin release PU/n-HA composite scaffolds reprogram macrophages for improved osteogenesis and angiogenesis
- PMID: 35415312
- PMCID: PMC8980440
- DOI: 10.1016/j.bioactmat.2022.03.037
Sequential gastrodin release PU/n-HA composite scaffolds reprogram macrophages for improved osteogenesis and angiogenesis
Abstract
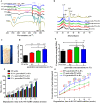
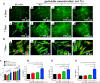
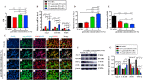
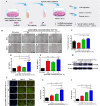
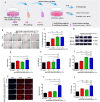
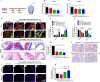
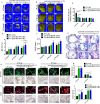
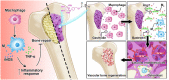
Wound healing is a highly orchestrated process involving a variety of cells, including immune cells. Developing immunomodulatory biomaterials for regenerative engineering applications, such as bone regeneration, is an appealing strategy. Herein, inspired by the immunomodulatory effects of gastrodin (a bioactive component in traditional Chinese herbal medicine), a series of new immunomodulatory gastrodin-comprising biodegradable polyurethane (gastrodin-PU) and nano-hydroxyapatite (n-HA) (gastrodin-PU/n-HA) composites were developed. RAW 264.7 macrophages, rat bone marrow mesenchymal stem cells (rBMSCs), and human umbilical vein endothelial cells (HUVECs) were cultured with gastrodin-PU/n-HA containing different concentrations of gastrodin (0.5%, 1%, and 2%) to decipher their immunomodulatory effects on osteogenesis and angiogenesis in vitro. Results demonstrated that, compared with PU/n-HA, gastrodin-PU/n-HA induced macrophage polarization toward the M2 phenotype, as evidenced by the higher expression level of pro-regenerative cytokines (CD206, Arg-1) and the lower expression of pro-inflammatory cytokines (iNOS). The expression levels of osteogenesis-related factors (BMP-2 and ALP) in the rBMSCs and angiogenesis-related factors (VEGF and BFGF) in the HUVECs were significantly up-regulated in gastrodin-PU/n-HA/macrophage-conditioned medium. The immunomodulatory effects of gastrodin-PU/n-HA to reprogram macrophages from a pro-inflammatory (M1) phenotype to an anti-inflammatory and pro-healing (M2) phenotype were validated in a rat subcutaneous implantation model. And the 2% gastrodin-PU/n-HA significantly decreased fibrous capsule formation and enhanced angiogenesis. Additionally, 2% gastrodin-PU/n-HA scaffolds implanted in the rat femoral condyle defect model showed accelerated osteogenesis and angiogenesis. Thus, the novel gastrodin-PU/n-HA scaffold may represent a new and promising immunomodulatory biomaterial for bone repair and regeneration.
Keywords: Angiogenesis; Gastrodin-delivery; Immune/inflammatory response; Osteogenesis; Tissue repair.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Microenvironment-optimized gastrodin-functionalized scaffolds orchestrate asymmetric division of recruited stem cells in endogenous bone regeneration.J Nanobiotechnology. 2024 Nov 20;22(1):722. doi: 10.1186/s12951-024-02886-7. J Nanobiotechnology. 2024. PMID: 39563380 Free PMC article.
-
Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells function.Bioact Mater. 2021 Jul 2;8:355-367. doi: 10.1016/j.bioactmat.2021.06.020. eCollection 2022 Feb. Bioact Mater. 2021. PMID: 34541406 Free PMC article.
-
Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner.Biomaterials. 2020 May;239:119833. doi: 10.1016/j.biomaterials.2020.119833. Epub 2020 Jan 31. Biomaterials. 2020. PMID: 32062479
-
Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies.Acta Biomater. 2021 Oct 1;133:4-16. doi: 10.1016/j.actbio.2021.03.038. Epub 2021 Mar 26. Acta Biomater. 2021. PMID: 33775905 Free PMC article. Review.
-
Immunomodulatory bioactive glasses for tissue regeneration.Acta Biomater. 2021 Oct 1;133:168-186. doi: 10.1016/j.actbio.2021.08.023. Epub 2021 Aug 18. Acta Biomater. 2021. PMID: 34418539 Review.
Cited by
-
3D printed shape-memory piezoelectric scaffolds with in-situ self-power properties for bone defect repair.J Nanobiotechnology. 2025 Mar 24;23(1):244. doi: 10.1186/s12951-025-03325-x. J Nanobiotechnology. 2025. PMID: 40128753 Free PMC article.
-
ROS-responsive adaptive injectable hydrogel promoting inflammatory mastoid bone repair through efficient sterilization and regulating oxidative stress and macrophage phenotype.Mater Today Bio. 2025 May 14;32:101856. doi: 10.1016/j.mtbio.2025.101856. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40496721 Free PMC article.
-
Silane-modified hydroxyapatite nanoparticles incorporated into polydioxanone/poly(lactide-co-caprolactone) creates a novel toughened nanocomposite with improved material properties and in vivo inflammatory responses.Mater Today Bio. 2023 Aug 24;22:100778. doi: 10.1016/j.mtbio.2023.100778. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37664796 Free PMC article.
-
One-step strategy for fabricating icariin-encapsulated biomimetic Scaffold: Orchestrating immune, angiogenic, and osteogenic cascade for enhanced bone regeneration.Bioact Mater. 2025 Jun 10;52:271-286. doi: 10.1016/j.bioactmat.2025.06.001. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40547322 Free PMC article.
-
Mechanism and Prospect of Gastrodin in Osteoporosis, Bone Regeneration, and Osseointegration.Pharmaceuticals (Basel). 2022 Nov 18;15(11):1432. doi: 10.3390/ph15111432. Pharmaceuticals (Basel). 2022. PMID: 36422561 Free PMC article. Review.
References
-
- Gouveia P.F., Mesquita-Guimarães J., Galárraga-Vinueza M.E., Souza J.C.M., Silva F.S., Fredel M.C., Boccaccini A.R., Detsch R., Henriques B. In-vitro mechanical and biological evaluation of novel zirconia reinforced bioglass scaffolds for bone repair. J. Mech. Behav. Biomed. Mater. 2021;114 doi: 10.1016/j.jmbbm.2020.104164. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

