Does Use of a Night Extension Orthosis Improve Outcomes in Patients With Dupuytren Contracture Treated With Injectable Collagenase?
- PMID: 35415567
- PMCID: PMC8991747
- DOI: 10.1016/j.jhsg.2021.05.001
Does Use of a Night Extension Orthosis Improve Outcomes in Patients With Dupuytren Contracture Treated With Injectable Collagenase?
Abstract
Purpose: Current prescribing information for the treatment of patients with Dupuytren contracture with injectable collagenase Clostridium histolyticum (CCH) recommends use of a night extension orthosis for 4 months after treatment. The present study examines whether this treatment improves the outcomes.
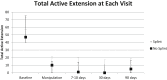
Methods: Adult patients with Dupuytren contracture treated with CCH during the study period were eligible for inclusion. The patients were randomized to orthosis or no orthosis groups and were stratified based on the severity of contracture prior to randomization. The orthosis group was fitted postmanipulation with a hand-based custom orthosis that held the treated finger in maximal comfortable extension, and the patients were instructed to wear the orthosis at night for 3 months. The patients were assessed at 7-10 days, 30 days, and 90 days postmanipulation. Orthosis compliance was measured with a survey. The primary outcome measure was improvement in total active extension (TAE), defined as the sum of active metacarpophalangeal (MCP), proximal interphalangeal, and distal interphalangeal joint extension in the treated finger at 90 days after treatment. Secondary outcomes included total active flexion (TAF), Michigan Hand Questionnaire scores, patient satisfaction, and clinical success.
Results: Twenty-six patients completed the study, 12 in the orthosis group and 14 in the no orthosis group. The majority of contractures (90%) were primarily through the MCP joint. The patients in both the groups demonstrated significant improvements in TAE at 90-day follow-up (orthosis P = .002, no orthosis P = .001) . The difference in improvement in the median TAE between the 2 groups was not significant (P = .40). There were no significant differences between groups for TAE, TAF, Michigan Hand Questionnaire scores, patient satisfaction, or clinical success at any of the time points assessed (P > .05).
Conclusions: In patients with Dupuytren contracture with primarily MCP joint involvement, providing an orthosis after treatment with CCH may not offer a short-term benefit compared with CCH treatment alone in terms of TAE, TAF, or patient-reported outcome measures.
Type of study/level of evidence: Therapeutic I.
Keywords: Collagenase; Dupuytren; Orthosis fabrication.
© 2021 The Authors.
Figures
References
-
- Badalamente M.A., Hurst L.C., Hentz V.R. Collagen as a clinical target: nonoperative treatment of Dupuytren’s disease. J Hand Surg Am. 2002;27(5):788–798. - PubMed
-
- Gilpin D., Coleman S., Hall S., Houston A., Karrasch J., Jones N. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand SurgAm. 2010;35(12):2027–2038.e1. - PubMed
-
- Auxilium Pharmaceuticals Inc 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125338s109lbl.pdf
-
- Hurst L.C., Badalamente M.A., Hentz V.R., et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. - PubMed
-
- Peimer C.A., Blazar P., Coleman S., et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am. 2013;38(1):12–22. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

