A 3D-printable device allowing fast and reproducible longitudinal preparation of mouse intestines
- PMID: 35415968
- PMCID: PMC9043725
- DOI: 10.1002/ame2.12228
A 3D-printable device allowing fast and reproducible longitudinal preparation of mouse intestines
Abstract
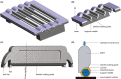
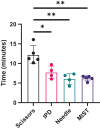
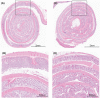
Accurate and reproducible analysis of murine small and large intestinal tissue is key for preclinical models involving intestinal pathology. Currently, there is no easily accessible, standardized method that allows researchers of different skill levels to consistently dissect intestines in a time-efficient manner. Here, we describe the design and use of the 3D-printed "Mouse Intestinal Slicing Tool" (MIST), which can be used to longitudinally dissect murine intestines for further analysis. We benchmarked the MIST against a commonly used procedure involving scissors to make a longitudinal cut along the intestines. Use of the MIST halved the time per mouse to prepare the intestines and outperformed alternative methods in smoothness of the cutting edge and overall reproducibility. By sharing the plans for printing the MIST, we hope to contribute a uniformly applicable method for saving time and increasing consistency in studies of the mouse gastrointestinal tract.
Keywords: adenoma; dissection; intestines; mice; printing, three-dimensional; reproducibility of results.
© 2022 The Authors. Animal Models and Experimental Medicine published by John Wiley & Sons Australia, Ltd on behalf of The Chinese Association for Laboratory Animal Sciences.
Conflict of interest statement
J.C. is a scientific advisor for Seed Health, Inc. Other authors do not have competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

