Generation of formaldehyde and formaldehyde-d2 for hydroxymethylations and hydroxydeuteromethylations of difluoroenolates and difluorobenzyl carbanions
- PMID: 35416212
- PMCID: PMC9205602
- DOI: 10.1039/d2cc01518h
Generation of formaldehyde and formaldehyde-d2 for hydroxymethylations and hydroxydeuteromethylations of difluoroenolates and difluorobenzyl carbanions
Abstract
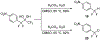
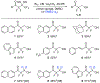
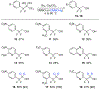
A method for the in situ production of formaldehyde from dimethylsulfoxide, bromine, and cesium carbonate is reported for reactions with difluoroenolates and difluorobenzyl carbanions. This process also generates formaldehyde-d2 for the production of 2,2-difluoro-1,1-deuteroethanols. Mechanistic and computational studies further characterize the production of hydroxymethylated and hydroxydeuteromethylated difluorinated organic molecules.
Conflict of interest statement
Conflicts of interest
D.A.C. serves as a scientific advisor for GnuPharma, and some of the studies in this manuscript were conducted with unrestricted support from GnuPharma. The authors declare no other competing financial interests.
Figures

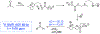
References
-
- Tokala R, Bora D, Sana S, Nachtigall FM, Santos LS and Shankaraiah N, J. Org. Chem, 2019, 84, 5504–5513. - PubMed
-
- Min L, Lin X and Li C-C, J. Am. Chem. Soc, 2019, 141, 15773–15778. - PubMed
-
- Boeckman RK Jr., Niziol JM and Biegasiewicz KF, Org. Lett, 2018, 20, 5062–5065. - PubMed
-
- Barcan GA, Conde JJ, Mokhallati MK, Nilson MG, Xie S, Alle CL, Andemichael YW, Calandra NA, Leitch DC, Li L and Morris MJ, Org. Process. Res. Dev, 2019, 23, 1396–1406.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

