A mesh-based model of liver vasculature: implications for improved radiation dosimetry to liver parenchyma for radiopharmaceuticals
- PMID: 35416550
- PMCID: PMC9008118
- DOI: 10.1186/s40658-022-00456-0
A mesh-based model of liver vasculature: implications for improved radiation dosimetry to liver parenchyma for radiopharmaceuticals
Abstract
Purpose: To develop a model of the internal vasculature of the adult liver and demonstrate its application to the differentiation of radiopharmaceutical decay sites within liver parenchyma from those within organ blood.
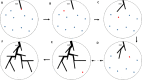
Method: Computer-generated models of hepatic arterial (HA), hepatic venous (HV), and hepatic portal venous (HPV) vascular trees were algorithmically created within individual lobes of the ICRP adult female and male livers (AFL/AML). For each iteration of the algorithm, pressure, blood flow, and vessel radii within each tree were updated as each new vessel was created and connected to a viable bifurcation site. The vascular networks created inside the AFL/AML were then tetrahedralized for coupling to the PHITS radiation transport code. Specific absorbed fractions (SAF) were computed for monoenergetic alpha particles, electrons, positrons, and photons. Dual-region liver models of the AFL/AML were proposed, and particle-specific SAF values were computed assuming radionuclide decays in blood within two locations: (1) sites within explicitly modeled hepatic vessels, and (2) sites within the hepatic blood pool residing outside these vessels to include the capillaries and blood sinuses. S values for 22 and 10 radionuclides commonly used in radiopharmaceutical therapy and imaging, respectively, were computed using the dual-region liver models and compared to those obtained in the existing single-region liver model.
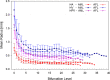
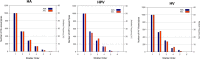
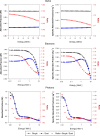
Results: Liver models with virtual vasculatures of ~ 6000 non-intersecting straight cylinders representing the HA, HPV, and HV circulations were created for the ICRP reference. For alpha emitters and for beta and auger-electron emitters, S values using the single-region models were approximately 11% (AML) to 14% (AFL) and 11% (AML) to 13% (AFL) higher than the S values obtained using the dual-region models, respectively.
Conclusions: The methodology employed in this study has shown improvements in organ parenchymal dosimetry through explicit consideration of blood self-dose for alpha particles (all energies) and for electrons at energies below ~ 100 keV.
Keywords: Hepatic vasculature; ICRP computational phantom; Liver; Radionuclide S values.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Bartlett RM, Bolch WE, Brill AB, Dewaraja YK, Fahey FH, Fisher DR, Hobbs RF, Howell RW, Meredith RF, Rajendran JG, Sgouros G, Zanzonico P. MIRD Primer 2020: A Complete Guide to Radiopharmaceutical Dosimetry. Reston, VA: The Society of Nuclear Medicine and Molecular Imaging (SNMMI); 2021.
-
- Sjogreen Gleisner K, Spezi E, Solny P, Gabina PM, Cicone F, Stokke C, Chiesa C, Paphiti M, Brans B, Sandstrom M, Tipping J, Konijnenberg M, Flux G. Variations in the practice of molecular radiotherapy and implementation of dosimetry: results from a European survey. EJNMMI Phys. 2017;4(1):28. doi: 10.1186/s40658-017-0193-4. - DOI - PMC - PubMed
-
- ICRU. ICRU Report 96: Dosimetry-guided radiopharmaceutical therapy. J ICRU. (in press).
Grants and funding
LinkOut - more resources
Full Text Sources

