X-ray irradiation triggers immune response in human T-lymphocytes via store-operated Ca2+ entry and NFAT activation
- PMID: 35416945
- PMCID: PMC9011325
- DOI: 10.1085/jgp.202112865
X-ray irradiation triggers immune response in human T-lymphocytes via store-operated Ca2+ entry and NFAT activation
Abstract
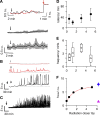
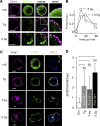
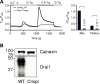
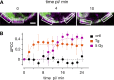
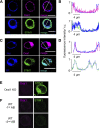
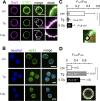
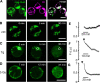
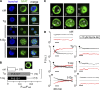
Radiation therapy efficiently eliminates cancer cells and reduces tumor growth. To understand collateral agonistic and antagonistic effects of this treatment on the immune system, we examined the impact of x-ray irradiation on human T cells. We find that, in a major population of leukemic Jurkat T cells and peripheral blood mononuclear cells, clinically relevant radiation doses trigger delayed oscillations of the cytosolic Ca2+ concentration. They are generated by store-operated Ca2+ entry (SOCE) following x-ray-induced clustering of Orai1 and STIM1 and formation of a Ca2+ release-activated Ca2+ (CRAC) channel. A consequence of the x-ray-triggered Ca2+ signaling cascade is translocation of the transcription factor nuclear factor of activated T cells (NFAT) from the cytosol into the nucleus, where it elicits the expression of genes required for immune activation. The data imply activation of blood immune cells by ionizing irradiation, with consequences for toxicity and therapeutic effects of radiation therapy.
© 2022 Tandl et al.
Figures


References
-
- Bauer, M., Goldstein M., Christmann M., Becker H., Heylmann D., and Kaina B.. 2011. Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress. Proc. Natl. Acad. Sci. USA. 108:21105–21110. 10.1073/pnas.1111919109 - DOI - PMC - PubMed

