Human pluripotent stem cell-derived kidney organoids for personalized congenital and idiopathic nephrotic syndrome modeling
- PMID: 35417019
- PMCID: PMC9148570
- DOI: 10.1242/dev.200198
Human pluripotent stem cell-derived kidney organoids for personalized congenital and idiopathic nephrotic syndrome modeling
Abstract
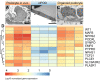
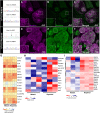
Nephrotic syndrome (NS) is characterized by severe proteinuria as a consequence of kidney glomerular injury due to podocyte damage. In vitro models mimicking in vivo podocyte characteristics are a prerequisite to resolve NS pathogenesis. The detailed characterization of organoid podocytes resulting from a hybrid culture protocol showed a podocyte population that resembles adult podocytes and was superior compared with 2D counterparts, based on single-cell RNA sequencing, super-resolution imaging and electron microscopy. In this study, these next-generation podocytes in kidney organoids enabled personalized idiopathic nephrotic syndrome modeling, as shown by activated slit diaphragm signaling and podocyte injury following protamine sulfate, puromycin aminonucleoside treatment and exposure to NS plasma containing pathogenic permeability factors. Organoids cultured from cells of a patient with heterozygous NPHS2 mutations showed poor NPHS2 expression and aberrant NPHS1 localization, which was reversible after genetic correction. Repaired organoids displayed increased VEGFA pathway activity and transcription factor activity known to be essential for podocyte physiology, as shown by RNA sequencing. This study shows that organoids are the preferred model of choice to study idiopathic and congenital podocytopathies.
Keywords: 2D iPSC-derived podocytes; Human iPSC-derived kidney organoids; Nephrotic syndrome.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
-
- Adam, R. C., Yang, H., Ge, Y., Infarinato, N. R., Gur-Cohen, S., Miao, Y., Wang, P., Zhao, Y., Lu, C. P., Kim, J. E.et al. (2020). NFI transcription factors provide chromatin access to maintain stem cell identity while preventing unintended lineage fate choices. Nat. Cell Biol. 22, 640-650. 10.1038/s41556-020-0513-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

