Inhibition of epigenetic reader proteins by apabetalone counters inflammation in activated innate immune cells from Fabry disease patients receiving enzyme replacement therapy
- PMID: 35417091
- PMCID: PMC9007222
- DOI: 10.1002/prp2.949
Inhibition of epigenetic reader proteins by apabetalone counters inflammation in activated innate immune cells from Fabry disease patients receiving enzyme replacement therapy
Abstract
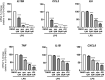
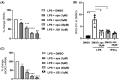
Fabry disease (FD) is a rare X-linked disorder of lipid metabolism, characterized by the accumulation of globotriaosylceramide (Gb3) due to defective the lysosomal enzyme, α-galactosidase. Gb3 deposits activate immune-mediated systemic inflammation, ultimately leading to life-threatening consequences in multiple organs such as the heart and kidneys. Enzyme replacement therapy (ERT), the standard of care, is less effective with advanced tissue injury and inflammation in patients with FD. Here, we showed that MCP-1 and TNF-α cytokine levels were almost doubled in plasma from ERT-treated FD patients. Chemokine receptor CCR2 surface expression was increased by twofold on monocytes from patients with low eGFR. We also observed an increase in IL12B transcripts in unstimulated peripheral blood mononuclear cells (PBMCs) over a 2-year period of continuous ERT. Apabetalone is a clinical-stage oral bromodomain and extra terminal protein inhibitor (BETi), which has beneficial effects on cardiovascular and kidney disease related pathways including inflammation. Here, we demonstrate that apabetalone, a BD2-selective BETi, dose dependently reduced the production of MCP-1 and IL-12 in stimulated PBMCs through transcriptional regulation of their encoding genes. Reactive oxygen species production was diminished by up to 80% in stimulated neutrophils following apabetalone treatment, corresponding with inhibition of NOX2 transcription. This study elucidates that inhibition of BET proteins by BD2-selective apabetalone alleviates inflammatory processes and oxidative stress in innate immune cells in general and in FD. These results suggest potential benefit of BD2-selective apabetalone in controlling inflammation and oxidative stress in FD, which will be further investigated in clinical trials.
Keywords: BETi; Fabry disease; apabetalone; epigenetics; inflammation; oxidative stress.
© 2022 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Figures





References
-
- Desnick RJ, Ioannou YA, Eng CM. α‐Galactosidase A deficiency: Fabry disease. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA, eds. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw‐Hill Education; 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

