Asymmetric Total Synthesis of (-)-Phaeocaulisin A
- PMID: 35417150
- PMCID: PMC9490872
- DOI: 10.1021/jacs.2c02188
Asymmetric Total Synthesis of (-)-Phaeocaulisin A
Abstract
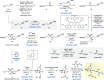
The therapeutic properties of Curcuma (ginger and turmeric's family) have long been known in traditional medicine. However, only recently have guaiane-type sesquiterpenes extracted from Curcuma phaeocaulis been submitted to biological testing, and their enhanced bioactivity was highlighted. Among these compounds, phaeocaulisin A has shown remarkable anti-inflammatory and anticancer activity, which appears to be tied to the unique bridged acetal moiety embedded in its tetracyclic framework. Prompted by the promising biological profile of phaeocaulisin A and by the absence of a synthetic route for its provision, we have implemented the first enantioselective total synthesis of phaeocaulisin A in 17 steps with 2% overall yield. Our route design builds on the identification of an enantioenriched lactone intermediate, tailored with both a ketone moiety and a conjugated alkene system. Taking advantage of the umpolung carbonyl-olefin coupling reactivity enabled by the archetypal single-electron transfer (SET) reductant samarium diiodide (SmI2), the lactone intermediate was submitted to two sequential SmI2-mediated cyclizations to stereoselectively construct the polycyclic core of the natural product. Crucially, by exploiting the innate inner-sphere nature of carbonyl reduction using SmI2, we have used a steric blocking strategy to render sites SET-unreceptive and thus achieve chemoselective reduction in a complex substrate. Our asymmetric route enabled elucidation of the naturally occurring isomer of phaeocaulisin A and provides a synthetic platform to access other guaiane-type sesquiterpenes from C. phaeocaulis─as well as their synthetic derivatives─for medicinal chemistry and drug design.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Prasad S.; Aggarwal B. B.. Herbal Medicine: Biomolecular and Clinical Aspects; 2nd editio.; Benzie I. F. F.; Wachtel-Galor S., Eds.; CRC Press/Taylor & Francis: 2011. - PubMed
-
- Committee on Herbal Medicinal European Medicines Agency Curcumae longae rhizoma; https://www.ema.europa.eu/en/medicines/herbal/curcumae-longae-rhizoma (accessed Jan 12, 2022).
-
- Ma G.-H.; Chen K.-X.; Zhang L.-Q.; Li Y.-M. Advance in Biological Activities of Natural Guaiane-Type Sesquiterpenes. Med. Chem. Res. 2019, 28, 1339–1358. 10.1007/s00044-019-02385-7. - DOI
- Lou Y.; Zhao F.; He H.; Peng K.-F.; Zhou X.-H.; Chen L.-X.; Qiu F. Guaiane-Type Sesquiterpenes from Curcuma Wenyujin and Their Inhibitory Effects on Nitric Oxide Production. J. Asian Nat. Prod. Res. 2009, 11, 737–747. 10.1080/10286020903042358. - DOI - PubMed
-
- Liu Y.; Ma J.; Zhao Q.; Liao C.; Ding L.; Chen L.; Zhao F.; Qiu F. Guaiane-Type Sesquiterpenes from Curcuma Phaeocaulis and Their Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2013, 76, 1150–1156. 10.1021/np400202f. - DOI - PubMed
- Yin G.-P.; Li L.-C.; Zhang Q.-Z.; An Y.-W.; Zhu J.-J.; Wang Z.-M.; Chou G.-X.; Wang Z. INOS Inhibitory Activity of Sesquiterpenoids and a Monoterpenoid from the Rhizomes of Curcuma Wenyujin. J. Nat. Prod. 2014, 77, 2161–2169. 10.1021/np400984c. - DOI - PubMed
-
- Lin T.Application of phaeocaulisin A in preparing medicine for treating melanoma. CN 2015, 105078967.
- Qiu F.; Liu Y.; Kang N.; Chen L.; Zhao F.; Wu B.. Sesquiterpenoids in zedoary as well as preparation method and application of sesquiterpenoids. CN2013, 103242275 A.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

