Resolving Oxygenation Pathways in Manganese-Catalyzed C(sp3)-H Functionalization via Radical and Cationic Intermediates
- PMID: 35417154
- PMCID: PMC9052745
- DOI: 10.1021/jacs.2c01466
Resolving Oxygenation Pathways in Manganese-Catalyzed C(sp3)-H Functionalization via Radical and Cationic Intermediates
Abstract
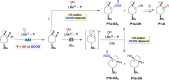
The C(sp3)-H bond oxygenation of the cyclopropane-containing mechanistic probes 6-tert-butylspiro[2.5]octane and spiro[2.5]octane with hydrogen peroxide catalyzed by manganese complexes bearing aminopyridine tetradentate ligands has been studied. Mixtures of unrearranged and rearranged oxygenation products (alcohols, ketones, and esters) are obtained, suggesting the involvement of cationic intermediates and the contribution of different pathways following the initial hydrogen atom transfer-based C-H bond cleavage step. Despite such a complex mechanistic scenario, a judicious choice of the catalyst structure and reaction conditions (solvent, temperature, and carboxylic acid) could be employed to resolve these oxygenation pathways, leading, with the former substrate, to conditions where a single unrearranged or rearranged product is obtained in good isolated yield. Taken together, the work demonstrates an unprecedented ability to precisely direct the chemoselectivity of the C-H oxidation reaction, discriminating among multiple pathways. In addition, these results conclusively demonstrate that stereospecific C(sp3)-H oxidation can take place via a cationic intermediate and that this path can become exclusive in governing product formation, expanding the available toolbox of aliphatic C-H bond oxygenations. The implications of these findings are discussed in the framework of the development of synthetically useful C-H functionalization procedures and the associated mechanistic features.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

