Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting
- PMID: 35417631
- PMCID: PMC9020581
- DOI: 10.1056/NEJMoa2201688
Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting
Abstract
Background: With large waves of infection driven by the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), alongside evidence of waning immunity after the booster dose of coronavirus disease 2019 (Covid-19) vaccine, several countries have begun giving at-risk persons a fourth vaccine dose.
Methods: To evaluate the early effectiveness of a fourth dose of the BNT162b2 vaccine for the prevention of Covid-19-related outcomes, we analyzed data recorded by the largest health care organization in Israel from January 3 to February 18, 2022. We evaluated the relative effectiveness of a fourth vaccine dose as compared with that of a third dose given at least 4 months earlier among persons 60 years of age or older. We compared outcomes in persons who had received a fourth dose with those in persons who had not, individually matching persons from these two groups with respect to multiple sociodemographic and clinical variables. A sensitivity analysis was performed with the use of parametric Poisson regression.
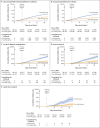
Results: The primary analysis included 182,122 matched pairs. Relative vaccine effectiveness in days 7 to 30 after the fourth dose was estimated to be 45% (95% confidence interval [CI], 44 to 47) against polymerase-chain-reaction-confirmed SARS-CoV-2 infection, 55% (95% CI, 53 to 58) against symptomatic Covid-19, 68% (95% CI, 59 to 74) against Covid-19-related hospitalization, 62% (95% CI, 50 to 74) against severe Covid-19, and 74% (95% CI, 50 to 90) against Covid-19-related death. The corresponding estimates in days 14 to 30 after the fourth dose were 52% (95% CI, 49 to 54), 61% (95% CI, 58 to 64), 72% (95% CI, 63 to 79), 64% (95% CI, 48 to 77), and 76% (95% CI, 48 to 91). In days 7 to 30 after a fourth vaccine dose, the difference in the absolute risk (three doses vs. four doses) was 180.1 cases per 100,000 persons (95% CI, 142.8 to 211.9) for Covid-19-related hospitalization and 68.8 cases per 100,000 persons (95% CI, 48.5 to 91.9) for severe Covid-19. In sensitivity analyses, estimates of relative effectiveness against documented infection were similar to those in the primary analysis.
Conclusions: A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19-related outcomes among persons who had received a third dose at least 4 months earlier. (Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Covid-19 Boosters - Where from Here?N Engl J Med. 2022 Apr 28;386(17):1661-1662. doi: 10.1056/NEJMe2203329. Epub 2022 Apr 13. N Engl J Med. 2022. PMID: 35417633 Free PMC article. No abstract available.
-
Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine.N Engl J Med. 2022 Jul 14;387(2):191. doi: 10.1056/NEJMc2206926. Epub 2022 Jun 29. N Engl J Med. 2022. PMID: 35767517 No abstract available.
References
-
- SARS-CoV-2 sequences by variant. Our World in Data (https://ourworldindata.org/grapher/covid-variants-bar?country=AUS~GBR~US...).
-
- Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Geneva: World Health Organization, November 26, 2021. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....).
-
- Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa. December 21, 2021. (https://www.medrxiv.org/content/10.1101/2021.12.21.21268116v1). preprint. - PMC - PubMed
-
- Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. Severity of omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. University of Edinburgh Research Explorer, December 22, 2021. (https://www.research.ed.ac.uk/en/publications/severity-of-omicron-varian...). - PMC - PubMed
-
- Effectiveness of 3 doses of COVID-19 vaccines against symptomatic COVID-19 and hospitalisation in adults aged 65 years and older. London: UK Health Security Agency, 2022. (https://khub.net/documents/135939561/338928724/Effectiveness+of+3+doses+...).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
