An evolutionary gap in primate default mode network organization
- PMID: 35417698
- PMCID: PMC9088817
- DOI: 10.1016/j.celrep.2022.110669
An evolutionary gap in primate default mode network organization
Abstract
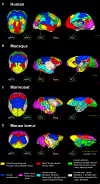
The human default mode network (DMN) is engaged at rest and in cognitive states such as self-directed thoughts. Interconnected homologous cortical areas in primates constitute a network considered as the equivalent. Here, based on a cross-species comparison of the DMN between humans and non-hominoid primates (macaques, marmosets, and mouse lemurs), we report major dissimilarities in connectivity profiles. Most importantly, the medial prefrontal cortex (mPFC) of non-hominoid primates is poorly engaged with the posterior cingulate cortex (PCC), though strong correlated activity between the human PCC and the mPFC is a key feature of the human DMN. Instead, a fronto-temporal resting-state network involving the mPFC was detected consistently across non-hominoid primate species. These common functional features shared between non-hominoid primates but not with humans suggest a substantial gap in the organization of the primate's DMN and its associated cognitive functions.
Keywords: BOLD; CP: Neuroscience; DMN; evolution; fMRI; macaque; marmoset; medial prefrontal cortex; mouse lemur; primates; resting-state.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Autio JA, Zhu Q, Li X, Glasser MF, Schwiedrzik CM, Fair DA, Zimmermann J, Yacoub E, Menon RS, Van Essen DC, et al. (2021). Minimal specifications for non-human primate MRI: challenges in standardizing and harmonizing data collection. NeuroImage 236, 118082. 10.1016/j.neuroimage.2021.118082. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

