Efficacy and Safety of Once-Weekly Somatrogon Compared with Once-Daily Somatropin (Genotropin®) in Japanese Children with Pediatric Growth Hormone Deficiency: Results from a Randomized Phase 3 Study
- PMID: 35417909
- PMCID: PMC9533447
- DOI: 10.1159/000524600
Efficacy and Safety of Once-Weekly Somatrogon Compared with Once-Daily Somatropin (Genotropin®) in Japanese Children with Pediatric Growth Hormone Deficiency: Results from a Randomized Phase 3 Study
Abstract
Introduction: Somatrogon is a long-acting recombinant human growth hormone being developed as a once-weekly treatment for children with growth hormone deficiency (GHD). The objective of this phase 3 study (NCT03874013) was to compare the efficacy and safety of once-weekly somatrogon with once-daily Genotropin in Japanese children with GHD.
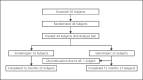
Methods: In this open-label, randomized, active-controlled study, 44 prepubertal Japanese children with GHD (boys: 3 to <11 years; girls: 3 to <10 years) were randomized 1:1 to receive once-weekly somatrogon or once-daily Genotropin (0.025 mg/kg/day) for 12 months. Dose escalation for somatrogon-treated subjects occurred in the first 6 weeks (0.25, 0.48, and 0.66 mg/kg/week; 2 weeks each) with the remaining 46 weeks at a dose of 0.66 mg/kg/week. The study's primary endpoint was annualized height velocity (HV) at 12 months.
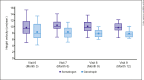
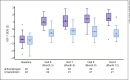
Results: Baseline characteristics were similar between treatment groups. Compared with Genotropin-treated subjects, somatrogon-treated subjects had higher least-squares mean HV at 12 months (9.65 cm/year vs. 7.87 cm/year). Once-weekly somatrogon was concluded as being comparable to once-daily Genotropin as the mean treatment difference (somatrogon-Genotropin) in HV was +1.79 cm/year (95% confidence interval, 0.97-2.61), which was greater than the preestablished margin (-1.8 cm/year). For both treatment groups, most adverse events were mild to moderate in severity and a similar proportion of subjects reported injection-site pain, although the somatrogon group reported more painful injections.
Conclusion: In prepubertal Japanese children with GHD, once-weekly somatrogon was comparable to once-daily Genotropin in terms of annualized (12-month) HV. Both treatments had similar safety and tolerability profiles.
Keywords: Genotropin; Growth hormone; Growth hormone deficiency; Long-acting growth hormone; Somatrogon.
© 2022 S. Karger AG, Basel.
Conflict of interest statement
R. Horikawa: advisory boards of Novo Nordisk, Pfizer, Lumos Pharma, and OPKO; consulting fees from Novo Nordisk, Pfizer, Lumos Pharma, Sandoz, and Ascendis Pharma; recipient of grants from Novo Nordisk and Sandoz; speakers bureau for Novo Nordisk, Pfizer, Eli Lilly & Company, and Sandoz. T. Tanaka: advisory board of OPKO and an Editorial Board Member of Hormone Research in Paediatrics. T. Yorifuji: advisory boards of Novo Nordisk and Pfizer; consulting fees from Novo Nordisk and Pfizer. R.G. Rosenfeld: advisory boards of Lumos, DNARx, and BioMarin; consulting fee from OPKO. Y. Hoshino, A. Okayama, D. Shima, and R. Gomez: employees and stockholders of Pfizer. A. Pastrak and O. Castellanos: employees and stockholders of OPKO. Y. Hasegawa and D. Ng: no conflicts of interest to declare.
Figures

References
-
- Richmond E, Rogol AD. Testing for growth hormone deficiency in children. Growth Horm IGF Res. 2020;50:57–60. - PubMed
-
- Rothermel J, Reinehr T. Metabolic alterations in paediatric GH deficiency. Best Pract Res Clin Endocrinol Metab. 2016;30((6)):757–70. - PubMed
-
- Collett-Solberg PF, Jorge AAL, Boguszewski MCS, Miller BS, Choong CSY, Cohen P, et al. Growth hormone therapy in children; research and practice: a review. Growth Horm IGF Res. 2019;44:20–32. - PubMed

