HIV oral self-screening test among HIV/STD/TB clinic attendees: A mixed-method pilot investigation examining merit for larger evaluation
- PMID: 35417993
- PMCID: PMC9707688
- DOI: 10.4103/ijmr.ijmr_3131_21
HIV oral self-screening test among HIV/STD/TB clinic attendees: A mixed-method pilot investigation examining merit for larger evaluation
Abstract
Background & objectives: Globally, several countries consider HIV self-test as an important element in the toolbox to end AIDS by 2030. Against this background, the present investigation was conducted to pilot test the performance of an indigenous HIV oral self-test (HIVOST) and explore its acceptability. The overall purpose was to examine if this kit could serve as a promising tool and merit future larger clinical evaluation.
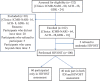
Methods: A concurrent mixed-method investigation was undertaken during March-October 2019. One hundred and thirty two consecutive HIV/sexually transmitted diseases/tuberculosis clinic attendees were invited for participation; of whom, 100 were enrolled, and among them, 40 provided consent for qualitative in-depth interviews. The HIVOST kit assessed for its performance served as the 'index test', which worked on the principle of lateral flow chromatography. The results of the HIVOST were interpreted independently by the study physicians and participants at 20 min. HIVOST kit performance was assessed against the HIV confirmatory blood test result based on the national algorithm (3 rapid test or 1 ELISA and 2 rapid test) serving as the 'reference'. Sensitivity, specificity, positive predictive value, negative predictive value and inter-rater agreement were estimated. The voices and concerns of the study participants were coded followed by identification of qualitative themes and ideas.
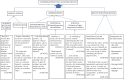
Results: The sensitivity and specificity of the index test at the end of 20 min as interpreted by the participants were 83.3 per cent [95% confidence interval (CI): 69.8 to 92.5] and 98 per cent (95% CI: 89.4 to 99.5), respectively. Study physicians and participants independently interpreted HIVOST results with substantial inter-rater agreement (kappa value 0.88; 95% CI: 0.78-0.97). All HIVOST test strips were valid. Majority of the participants preferred saliva over blood for HIV self-test. 'Comfort', 'confidentiality' and 'convenience' were the perceived advantages of HIVOST. Some of the participants wished the package inserts contained 'how-to-do instructions in local languages', 'expiry date (if any)' and 'contact helpline number'. A few of them highlighted the need for a confirmatory HIV result following oral self-test. Concerns of the participants revolved around potential self-harm following HIVOST-positive result and safe disposal of kits.
Interpretation & conclusions: Two major highlights of the present investigation are (i) high level of concordance in HIVOST results interpreted by participants and physicians, and (ii) encouraging level of acceptance of HIVOST. These findings and encouraging HIVOST performance statistics lend support towards large-scale clinical evaluation of this index test.
Keywords: HIV; India; indigenous HIV oral-self-test; mixed-method investigation; self-screening.
Conflict of interest statement
Figures
References
-
- The Joint United Nations Programme on HIV/AIDS (UNAIDS) Fast-Track –Ending the AIDS epidemic by 2030. [accessed on March 11, 2021]. Available from: https://www.unaids.org/sites/default/files/media_asset/jc2686_wad2014rep... .
-
- World Health Organization. Guidelines on HIV self-testing and partner notification: Supplement to consolidated guidelines on HIV testing services. Geneva: WHO; 2016. - PubMed
-
- National AIDS Control Organization. National strategic plan for HIV/AIDS and STI 2017-2024: Paving way for an AIDS free India. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2017.
-
- National AIDS Control Organization, ICMR-National Institute of Medical Statistics. HIV estimations 2017: Technical report. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2018.
-
- International Centre for AIDS Care and Treatment Program. ICAP Approach to Strategic HIV Testing. Columbia University. [accessed on January 6, 2020]. Available from: https://icap.columbia.edu/wpcontent/uploads/ICAP_Approach_to_Strategic_H... .
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials