Targeting CD47/SIRPα as a therapeutic strategy, where we are and where we are headed
- PMID: 35418166
- PMCID: PMC9009010
- DOI: 10.1186/s40364-022-00373-5
Targeting CD47/SIRPα as a therapeutic strategy, where we are and where we are headed
Abstract
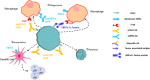
Immunotherapy using PD-1 and CTLA4 inhibitors to stimulate T cell immunity has achieved significant clinical success. However, only a portion of patients benefit from T cell-based immunotherapy. Macrophages, the most abundant type of innate immune cells in the body, play an important role in eliminating tumor cells and infectious microbes. The phagocytic check point protein CD47 inhibits the phagocytic activity of macrophages through binding to SIRPα expressed on macrophages. Blockade of the interaction between CD47 and SIRPα could restore phagocytic activity and eliminate tumor cells in vitro and in vivo. In this manuscript, we review the mechanism of action and development status of agents (antibodies targeting CD47 and SIRPα, SIRPα-Fc fusion proteins, and bi-specific antibodies) that block CD47/SIRPα interaction in preclinical studies and in the clinical setting. In addition, small molecules, mRNA, and CAR-T/M that target the CD47/SIRPα axis are also reviewed in this article.
Keywords: Bispecific antibody; CD47; Clinical development; Immunotherapy; Phagocytosis; SIRPα.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

