Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia
- PMID: 35418180
- PMCID: PMC9007937
- DOI: 10.1038/s41408-022-00660-2
Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia
Abstract
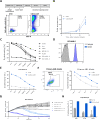
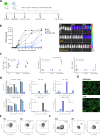
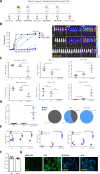
Acute myeloid leukemia (AML) is a malignant disorder derived from neoplastic myeloid progenitor cells characterized by abnormal proliferation and differentiation. Although novel therapeutics have recently been introduced, AML remains a therapeutic challenge with insufficient cure rates. In the last years, immune-directed therapies such as chimeric antigen receptor (CAR)-T cells were introduced, which showed outstanding clinical activity against B-cell malignancies including acute lymphoblastic leukemia (ALL). However, the application of CAR-T cells appears to be challenging due to the enormous molecular heterogeneity of the disease and potential long-term suppression of hematopoiesis. Here we report on the generation of CD33-targeted CAR-modified natural killer (NK) cells by transduction of blood-derived primary NK cells using baboon envelope pseudotyped lentiviral vectors (BaEV-LVs). Transduced cells displayed stable CAR-expression, unimpeded proliferation, and increased cytotoxic activity against CD33-positive OCI-AML2 and primary AML cells in vitro. Furthermore, CD33-CAR-NK cells strongly reduced leukemic burden and prevented bone marrow engraftment of leukemic cells in OCI-AML2 xenograft mouse models without observable side effects.
© 2022. The Author(s).
Conflict of interest statement
RP, MN, MQ, CZ, and NM are employees of Miltenyi Biotec. DS is employee of Lentigen Technology, Inc., a Miltenyi Biotec Company. MWMK is a consultant for Pfizer and Abbvie and receives travel support from Celgene and Daiichi Sankyo. OP and EU have no COIs directly related to this manuscript. OP has received honoraria or travel support from Astellas, Gilead, Jazz, MSD, Neovii Biotech, Novartis, Pfizer, and Therakos. He has received research support from Gilead, Incyte, Jazz, Neovii Biotech, and Takeda and is member of advisory boards to Jazz, Gilead, MSD, Omeros, Priothera, Shionogi, and SOBI. EU has a sponsored research project with Gilead. Remaining authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

