Deletion of activin A in mesenchymal but not myeloid cells ameliorates disease severity in experimental arthritis
- PMID: 35418478
- PMCID: PMC9279851
- DOI: 10.1136/annrheumdis-2021-221409
Deletion of activin A in mesenchymal but not myeloid cells ameliorates disease severity in experimental arthritis
Abstract
Objective: The aim of this study was to assess the extent and the mechanism by which activin A contributes to progressive joint destruction in experimental arthritis and which activin A-expressing cell type is important for disease progression.
Methods: Levels of activin A in synovial tissues were evaluated by immunohistochemistry, cell-specific expression and secretion by PCR and ELISA, respectively. Osteoclast (OC) formation was assessed by tartrat-resistant acid phosphatase (TRAP) staining and activity by resorption assay. Quantitative assessment of joint inflammation and bone destruction was performed by histological and micro-CT analysis. Immunoblotting was applied for evaluation of signalling pathways.
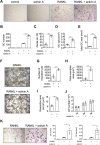
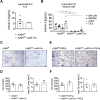
Results: In this study, we demonstrate that fibroblast-like synoviocytes (FLS) are the main producers of activin A in arthritic joints. Most significantly, we show for the first time that deficiency of activin A in arthritic FLS (ActβAd/d ColVI-Cre) but not in myeloid cells (ActβAd/d LysM-Cre) reduces OC development in vitro, indicating that activin A promotes osteoclastogenesis in a paracrine manner. Mechanistically, activin A enhanced OC formation and activity by promoting the interaction of activated Smad2 with NFATc1, the key transcription factor of osteoclastogenesis. Consistently, ActβAd/d LysM-Cre hTNFtg mice did not show reduced disease severity, whereas deficiency of activin A in ColVI-Cre-expressing cells such as FLS highly diminished joint destruction reflected by less inflammation and less bone destruction.
Conclusions: The results highly suggest that FLS-derived activin A plays a crucial paracrine role in inflammatory joint destruction and may be a promising target for treating inflammatory disorders associated with OC formation and bone destruction like rheumatoid arthritis.
Keywords: arthritis, rheumatoid; fibroblasts; inflammation; rheumatoid Arthritis.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





Similar articles
-
Activin A induces cell proliferation of fibroblast-like synoviocytes in rheumatoid arthritis.Arthritis Rheum. 2003 Sep;48(9):2442-9. doi: 10.1002/art.11249. Arthritis Rheum. 2003. PMID: 13130463
-
Sonic hedgehog promotes synovial inflammation and articular damage through p38 mitogen-activated protein kinase signaling in experimental arthritis.J Autoimmun. 2022 Oct;132:102902. doi: 10.1016/j.jaut.2022.102902. Epub 2022 Sep 8. J Autoimmun. 2022. PMID: 36088884
-
SMAD2 inhibits pyroptosis of fibroblast-like synoviocytes and secretion of inflammatory factors via the TGF-β pathway in rheumatoid arthritis.Arthritis Res Ther. 2023 Aug 9;25(1):144. doi: 10.1186/s13075-023-03136-1. Arthritis Res Ther. 2023. PMID: 37559090 Free PMC article.
-
MicroRNA-17-5p Reduces Inflammation and Bone Erosions in Mice With Collagen-Induced Arthritis and Directly Targets the JAK/STAT Pathway in Rheumatoid Arthritis Fibroblast-like Synoviocytes.Arthritis Rheumatol. 2020 Dec;72(12):2030-2039. doi: 10.1002/art.41441. Epub 2020 Oct 29. Arthritis Rheumatol. 2020. PMID: 32683798
-
Where to Stand with Stromal Cells and Chronic Synovitis in Rheumatoid Arthritis?Cells. 2019 Oct 15;8(10):1257. doi: 10.3390/cells8101257. Cells. 2019. PMID: 31618926 Free PMC article. Review.
Cited by
-
Single-cell transcriptomics of human cholesteatoma identifies an activin A-producing osteoclastogenic fibroblast subset inducing bone destruction.Nat Commun. 2023 Aug 3;14(1):4417. doi: 10.1038/s41467-023-40094-3. Nat Commun. 2023. PMID: 37537159 Free PMC article.
-
Neutrophil-derived Activin-A moderates their pro-NETotic activity and attenuates collateral tissue damage caused by Influenza A virus infection.Front Immunol. 2024 Feb 26;15:1302489. doi: 10.3389/fimmu.2024.1302489. eCollection 2024. Front Immunol. 2024. PMID: 38476229 Free PMC article.
-
In chronic kidney disease altered cardiac metabolism precedes cardiac hypertrophy.Am J Physiol Renal Physiol. 2024 May 1;326(5):F751-F767. doi: 10.1152/ajprenal.00416.2023. Epub 2024 Feb 22. Am J Physiol Renal Physiol. 2024. PMID: 38385175 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

