Prolonged viral suppression with anti-HIV-1 antibody therapy
- PMID: 35418681
- PMCID: PMC9177424
- DOI: 10.1038/s41586-022-04597-1
Prolonged viral suppression with anti-HIV-1 antibody therapy
Abstract
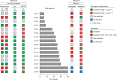
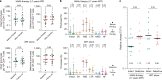
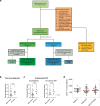
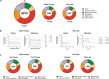
HIV-1 infection remains a public health problem with no cure. Anti-retroviral therapy (ART) is effective but requires lifelong drug administration owing to a stable reservoir of latent proviruses integrated into the genome of CD4+ T cells1. Immunotherapy with anti-HIV-1 antibodies has the potential to suppress infection and increase the rate of clearance of infected cells2,3. Here we report on a clinical study in which people living with HIV received seven doses of a combination of two broadly neutralizing antibodies over 20 weeks in the presence or absence of ART. Without pre-screening for antibody sensitivity, 76% (13 out of 17) of the volunteers maintained virologic suppression for at least 20 weeks off ART. Post hoc sensitivity analyses were not predictive of the time to viral rebound. Individuals in whom virus remained suppressed for more than 20 weeks showed rebound viraemia after one of the antibodies reached serum concentrations below 10 µg ml-1. Two of the individuals who received all seven antibody doses maintained suppression after one year. Reservoir analysis performed after six months of antibody therapy revealed changes in the size and composition of the intact proviral reservoir. By contrast, there was no measurable decrease in the defective reservoir in the same individuals. These data suggest that antibody administration affects the HIV-1 reservoir, but additional larger and longer studies will be required to define the precise effect of antibody immunotherapy on the reservoir.
© 2022. The Author(s).
Conflict of interest statement
There are patents on 3BNC117 (PTC/US2012/038400) and 10-1074 (PTC/US2013/065696) that list M.C.N. and J.F.S. as inventors. 3BNC117 and 10-1074 are licensed to Gilead by Rockefeller University from which M.C.N. and J.F.S. have received payments. M.C.N. is a member of the Scientific Advisory Boards of Celldex, Walking Fish, and Frontier Biotechnologies. J.F.S. and M.C.N had no control over the direction, and ultimately the reporting, of the clinical portion of the research while holding their financial interests, which were reviewed and are managed by the Rockefeller University and Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. J.D.R and C.J.P. are employees of Labcorp-Monogram Biosciences.
Figures







Comment in
-
Combination anti-HIV antibodies to achieve antiretroviral therapy-free virological suppression in infected individuals.Clin Transl Med. 2022 Sep;12(9):e1057. doi: 10.1002/ctm2.1057. Clin Transl Med. 2022. PMID: 36101965 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

