H2 and carbon-heteroatom bond activation mediated by polarized heterobimetallic complexes
- PMID: 35418712
- PMCID: PMC9004596
- DOI: 10.1016/j.ccr.2020.213765
H2 and carbon-heteroatom bond activation mediated by polarized heterobimetallic complexes
Abstract
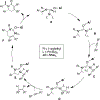
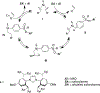
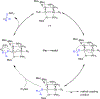
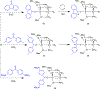
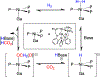
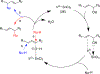
The field of heterobimetallic chemistry has rapidly expanded over the last decade. In addition to their interesting structural features, heterobimetallic structures have been found to facilitate a range of stoichiometric bond activations and catalytic processes. The accompanying review summarizes advances in this area since January of 2010. The review encompasses well-characterized heterobimetallic complexes, with a particular focus on mechanistic details surrounding their reactivity applications.
Keywords: Bond activation; Catalysis; Heterobimetallic; Metalloligand.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





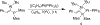




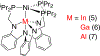

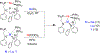

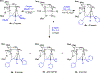

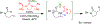





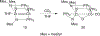


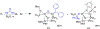


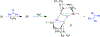









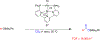

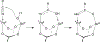
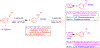




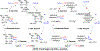
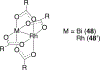

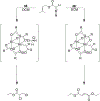

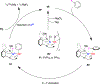

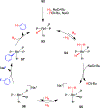



References
-
- Barden BA, Culcu G, Krogman JP, Bezpalko MW, Hatzis GP, Dickie DA, Foxman BM, Thomas CM, Assessing the Metal-Metal Interactions in a Series of Heterobimetallic Nb/M Complexes (M = Fe Co, Ni, Cu) and Their Effect on Multielectron Redox Properties, Inorg. Chem 58 (1) (2019) 821–833, 10.1021/acs.inorgchem.8b02960. - DOI - PubMed
-
- Bodio E, Picquet M, Le Gendre P, “Early–Late” Heterobimetallic Catalysis and Beyond, in: Kalck P (Ed.), Homo- and Heterobimetallic Complexes in Catalysis: Cooperative Catalysis, Springer International Publishing, Cham, 2016, pp. 139–186, 10.1007/3418_2015_161. - DOI
-
- Böhmer M, Guisado-Barrios G, Kampert F, Roelfes F, Tan TTY, Peris E, Hahn FE, Synthesis and catalytic applications of heterobimetallic carbene complexes obtained via sequential metalation of two bisazolium salts, Organometallics 38 (9) (2019) 2120–2131, 10.1021/acs.organomet.9b00120. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
