Subthalamic Deep Brain Stimulation Lead Asymmetry Impacts the Parkinsonian Gait Disorder
- PMID: 35418844
- PMCID: PMC8995434
- DOI: 10.3389/fnhum.2022.788200
Subthalamic Deep Brain Stimulation Lead Asymmetry Impacts the Parkinsonian Gait Disorder
Abstract
Background: The preferable position of Deep Brain Stimulation (DBS) electrodes is proposed to be located in the dorsolateral subthalamic nucleus (STN) to improve general motor performance. The optimal DBS electrode localization for the post-operative improvement of balance and gait is unknown.
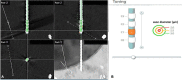
Methods: In this single-center, retrospective analyses, 66 Parkinson's disease (PD) patients (24 female, age 63 ± 7 years) were assessed pre- and post-operatively (8.45 ± 4.2 months after surgery) by using MDS-UPDRS, freezing of gait (FoG) score, Giladi's gait and falls questionnaire and Berg balance scale. The clinical outcome was related to the DBS electrode coordinates in x, y, z plane as revealed by image-based reconstruction (SureTune™). Binomial generalized linear mixed models with fixed-effect variables electrode asymmetry, parkinsonian subtype, medication, age class and clinical DBS induced changes were analyzed.
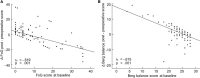
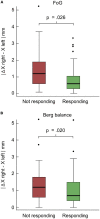
Results: Subthalamic nucleus-deep brain stimulation improved all motor, balance and FoG scores in MED OFF condition, however there were heterogeneous results in MED ON condition. DBS electrode reconstructed coordinates impacted the responsiveness of axial symptoms. FoG and balance responders showed slightly more medially located STN electrode coordinates and less medio-lateral asymmetry of the electrode reconstructed coordinates across hemispheres compared to non-responders.
Conclusion: Deep brain stimulation electrode reconstructed coordinates, particularly electrode asymmetry on the medio-lateral axis affected the post-operative responsiveness of balance and FoG symptoms in PD patients.
Keywords: Parkinson’s disease; balance; deep brain stimulation; electrode localization; freezing of gait; gait disorder; lead asymmetry; subthalamic nucleus.
Copyright © 2022 Schott, Gulberti, Pinnschmidt, Gerloff, Moll, Schaper, Koeppen, Hamel and Pötter-Nerger.
Conflict of interest statement
AG, CM, and WH had occasionally been reimbursed for travel expenses from Medtronic Inc. CG reported personal fees and other from Bayer Healthcare and Boehringer Ingelheim, personal fees from Abbott, Amgen, BMS, Sanofi Aventis, and Prediction Biosciences. CM received lecture, teaching, and proctoring fees from Abbott. WH received lecture fees and honoraria for serving on advisory boards and travel grants from Boston Scientific, Medtronic, and Abbott. MP-N received lecture fees from Abbott and Licher, and served as consultant for Medtronic, Boston Scientific, and Abbvie. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

